About our project
The Problem
Over 500,000 cardiac surgeries are performed in the US every year that require blood transfusions. The blood used in these transfusions is often stored for over 2 weeks before entering the patient’s body, during this period, over 20% of the blood deteriorates. The damaged blood being used contains toxins released by the irreversibly damaged red blood cells.
Transfusing this damaged blood can lead to:
• Increased risk of infection
• Organ damage
• Mortality
Our Solution
Ex Vivo Dynamics is developing a new medical filter technology (BloodGuardTM) that will reduce complications, readmission rates, and mortality in patients’ receiving transfusions during cardiac surgery by filtering toxins in the transfused blood before it enters the patient’s body.
How it Works
BloodGuardTM is a medical filter that attaches to the blood unit and removes the irreversibly damaged red blood cells and toxins before they enter the patient’s body. The filter allows only the viable red blood cells to enter the patient’s body, thereby significantly reducing most of the current transfusion-related complications.
BloodGuard
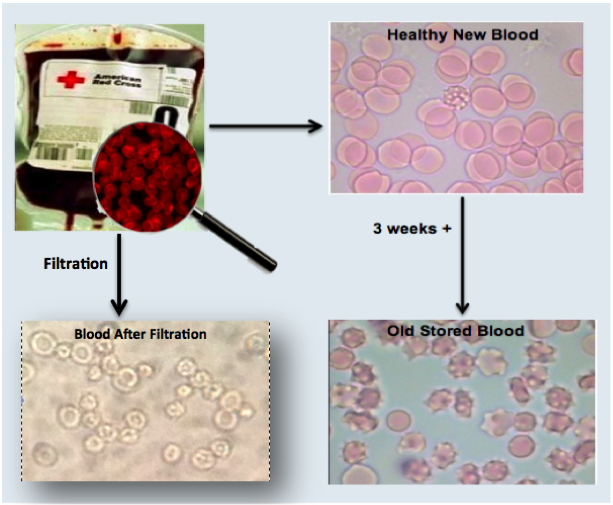
Microscopic Image of Blood Before and After Filtration
Overview of Blood Filtration Through the Filter

Clinical Proof to Date
We have conducted successful lab testing of our filter prototypes with blood analogs and human blood in filtering the damaged RBC’s and oxidative components. Currently we are focused on obtaining data to submit for government grants to help us advance the technology towards human trials and FDA approval.
FDA Status
We are in the preclinical phase of our product development.
How your project will help reduce cardiovascular disease and stroke by 20% by 2020
BloodGuard will significantly improve the cardiac landscape in two key ways:
• Reduce the damage in heart tissue in patients requiring large numbers of blood transfusions like anemia, thalassemia, and leukemia that increase the rate of cardiovascular disease.
• Improve the outcomes of patients receiving blood transfusions during cardiac procedures by giving patients safer, cleaner, and filtered blood.
How We Help Patients
BloodGuardTM will drastically improve the safety of cardiac procedures that require blood transfusions by ensuring only safe, clean, and filtered blood enters the patient’s body. BloodGuard removes toxins in the blood, allowing only healthy blood cells to enter the patient’s body. This will increase patient recovery time, lower out of pocket costs and result in higher survival rates.
How We Help Doctors
Our product is a cost-effective and safe way for doctors to drastically improve the outcome of transfusion in cardiac procedures.
Providing safer quality blood we can enable doctors to:
• Prevent complications during and post cardiac procedures
• Increase recovery and survival rate of patients
• Reduce additional treatments and cost
How We Help Hospitals, Institutions, and/ or Medical Facilities
Medicaid and Medicare fine hospitals due to additional treatment costs arising from complications from treatment procedures.
Our technology will help in:
• Faster patient recovery and patient discharge.
• Lower patient readmission, thus reduce healthcare cost.
• Prevent fines charged by Medicaid & Medicare.
• Help hospitals provide a better quality of treatment to patients and reduce healthcare cost.
How We Help Our Partners
We are developing a new technology for an unexplored niche that will change the practice of transfusion medicine and improve the standard of care. This presents many possibilities for our partners to leverage our technology and provide better quality medical care for all.
How We Will Spend Your Contributions
Contributions will be used to help our company advance BloodGuard in the following ways:
• Improve our prototype
• Conduct advanced testing in preclinical studies
• Conduct and collect data from advanced clinical studies
• FDA approval and IRB approval filings
- All donations will be used for product development cost. No salaries will be drawn from these funds by Ex Vivo Dynamics.*
Our Team
Ex Vivo Dynamics Management Team has skilled professionals with expertise in both research and business development. We have mentors and advisors with expertise in transfusion medicine, medical devices, organic chemistry and business and strategic development to help and guide us through the development of our product and our company.
Founder & CEO – Alan Perlstein, M.S

Role: Business and Product Development
Alan Perlstein has 10 years experience in research and development in biologics and devices.
LinkedIn
Chief Scientific Officer – Dr. Xi Huang, MS, PhD

Role: Research & Product Development
Dr. Xi Huang has more than 20 years experience in studying iron physiology and toxicology and has developed sensitive fluorescent assay to measure toxic iron and validated several reliable iron markers for evaluating iron status in patients. He will be working on the product development, preclinical and clinical studies.
LinkedIn
Chief Administrative Officer – Daisy Lobo, M.S

Role: Business Development
Daisy has experience in research, business and strategic development of healthcare and biotechnology companies and will be working with the business related duties of the company and research activities of product development.
LinkedIn
Chief Business Officer – Mei Mei Zhao, B.A

Role: Business & Marketing Development
Mei-Mei has worked and held significant leadership roles in strategy consulting, venture capital, and business strategy and marketing in healthcare.
LinkedIn
Business Development – Ivy Saludes, MS, MBA

Role: Reimbursement and Strategy Development
She has nearly ten years of professional experience advising start-ups and established companies in their growth strategies, strategic partnerships and capital raising.
LinkedIn
How to Find Us
Website
References:
1) E.A. Hod , S.L. Spitalnik; 7 June 2012; Stored red blood cell transfusions: Iron, inflammation, immunity, and infection; Transfus Clin Biol. 19 (2012) 84–89
2) Eldad A. Hod, Steven L. Spitalnik; 15 APR 2011; Harmful effects of transfusion of older stored red blood cells: iron and inflammation; DOI: 10.1111/j.1537-2995.2011.03096.x
3) Niels Liona,b,⁎, David Crettaza, Olivier Rubina, Jean-Daniel Tissota 4 November 2009, Stored red blood cells: A changing universe waiting for its map(s); J Proteomics, 2010 Jan 3;73(3):374-85
4) Eldad A. Hod, Gary M. Brittenham, Genia B. Billote, Richard O. Francis, Yelena Z. Ginzburg, Jeanne E. Hendrickson, Jeffrey Jhang, Joseph Schwartz, Shruti Sharma, Sujit Sheth, Anthony N. Sireci, Hannah L. Stephens, Brie A. Stotler, Boguslaw S. Wojczyk, James C. Zimring and Steven L. Spitalnik; October 20, 2011; Transfusion of human volunteers with older, stored red blood cells produces extravascular hemolysis and circulating non − transferrin-bound iron; Blood. 2011 Dec 15;118(25):6675-82
5) Eldad A. Hod, Ning Zhang, Set A. Sokol, Boguslaw S. Wojczyk, Richard O. Francis, Daniel Ansaldi, Kevin P. Francis, Phyllis Della-Latta, Susan Whittier, Sujit Sheth, Jeanne E. Hendrickson, James C. Zimring, Gary M. Brittenham and Steven L. Spital; March 18, 2010; Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation; Blood. 2010 May 27;115(21):4284-92
5) Caroline P. Ozment, Lisa B. Mamo, Mary Lee Campbell, Yuliya Lokhnygina, Andrew J. Ghio, and Jennifer L. Turi; April 2011; Transfusion-related biologic effects and free hemoglobin, heme, and iron;
DOI: 10.1111/j.1537-2995.2011.03096.x
6) Pradeep Gujja, M.D., Douglas R. Rosing, M.D., […], and Yukitaka Shizukuda, M.D., PhD, F.A.C.C; 2010; Iron Overload Cardiomyopathy, Better Understanding of An Increasing Disorder; doi: 10.1016/j.jacc.2010.03.083
7) Sowemimo-Coker SO; 2002; Red blood cell hemolysis during processing; Transfus Med Rev. 2002 Jan;16(1):46-60.
-
Have a question?
If the info above does not help, you can ask the project creator directly.
-
-