Campaign to Cure HIV: Complete Pre-Clinical Testing of VS2-370 CDK4, 6 and 9 Inhibtor

We are seeking $1.5 Million in donations to further development of a novel drug providing a functional cure for HIV and for HIV-associated malignancies.
Colorado Springs, CO United States Drug Development MedStartr Ventures challengeAbout our project
The problem we solve: Current therapies for treating HIV do prevent the infection of new cells, but do not reduce the HIV reservoir. The result is that HIV is never eliminated from the body exposing AIDS patients to high risk to develop HIV-associated malignancies. In addition, there is no clear pathway for any HIV “Cure” product currently under development to obtain regulatory approval if the product is HIV specific.
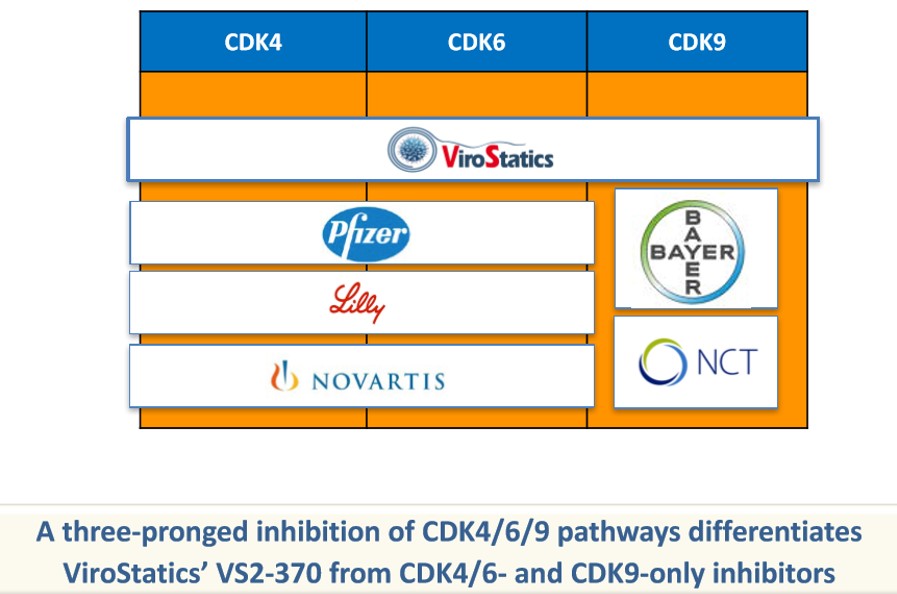
About our solution: VS2-370 is the only CDK 4/6/9 inhibitor currently in development and the preclinical studies conducted thus far have shown that VS2-370 not only is efficacious against certain cancers, many of which afflict those suffering with AIDS, but also appears to purge HIV “reservoir” cells. Consequently VS2-370 would provide a functional HIV cure. In addition, because of the dual indication of VS2-370, there is a clear pathway to obtain regulatory approval, initially as a cancer treatment and then subsequently as a functional HIV cure.
Progress to date:
We have shown in the laboratory and in animal experiments that VS2-370 is unique, selective, active and safe.
Consistent with its target and mechanism of action, VS2-370 is not only active against several aggressive tumors, including lymphomas and pancreatic cancer, but also against chronic viruses and affects all HIV reservoir-sustaining pathways with the potential for HIV Cure. For these reasons we propose that the initial indication be an HIV associated malignancy, such as primary effusion lymphoma (PEL), a rare and aggressive B-cell non-Hodgkin's lymphoma that usually presents with malignant effusions without tumor masses. PEL is resistant to chemotherapy (no standard of care has been established) with a short median survival of less than 6 months, representing a high unmet medical need. PEL is very sensitive to VS2-370 in vitro and in vivo/animal model. A key advantage of VS2-370 over other HIV Cure compounds that are under development is a clear pathway to obtain regulatory approval for cancer treatment, to be subsequently extended to the HIV Cure.
Aim of the present project is to complete the preclinical development of VS2-370 by:
o Concluding in vitro stability and bioavailability studies (already collected promising initial stability exposure profile data in vivo)
o Performing Good Laboratory Practice (GLP) toxicology studies in rodents and non-rodents (we have already collected Maximal Tolerated Dose –MTD– and Dose Range Finding –DRF– non-GLP data in the same species)
o Writing an Investigational Medicinal Product Dossier –IMPD– that is essential to obtain green light to move to clinical trial by the regulatory authorities (we have already obtained EMA scientific advices)
o Synthesizing a VS2-370 clinical batch (we presently have 1 Kg material for preclinical GLP studies)
We have already obtained an encouraging initial feedback on our program from potential Pharma partners. Our platform pipeline bears the potential to expand to other indications backed up by solid available data (e.g. pancreatic cancer, non-small cell lung cancer, leukemias)
About Our Team
Creator: Michael Richards
Location: Colorado
Bio: Fundraising Strategist/Coordinator for the Research Institute for Genetic and Human Therapy (RIGHT)
Hospital Affiliation: Research Institute for Genetic and Human Therapy (RIGHT)
Title: Fundraising Strategist/Coordinator
About Team Members
Rebecca Hautman
Executive Director,
Biography: Executive Director for the Research Institute for Genetic and Human Therapy (RIGHT)
Title: Executive Director
John Hautman
President,
Biography: President for the Research Institute for Genetic and Human Therapy (RIGHT)
Title: President
About Our Company
Research Institute for Genetic and Human Therapy (RIGHT)
Location: 6555 Ashton Park Place
Colorado Springs, CO 80919
US
Founded: 1995
Website: http://rightinstitute.net/
Facebook: https://www.facebook.com/groups/246679959186858/
Other link: http://www.virostatics.com/
Product Stage: Prototype/MVP
YTD Sales: Less than $250,000
Employees: 3-5
Innovation Details
Intellectual Property Summary
VS2-370 is covered by a composition of matter patent application (WO 2014031937 A1 filed August 2013) which has been allowed in the US, is in active prosecution in the European Patent Office, Eurasia, Australia, China, Israel, New Zealand, South Africa, and Thailand, and has also been filed in Ukraine, ARIPO, Brazil, Canada, Hong Kong, India, Indonesia, Japan, S. Korea, Mexico, Nigeria, Singapore. ViroStatics is the owner.
Patent Link
http://www.google.com/patents/WO2014031937A1?cl=en
Clinical Information
VS2-370 is completing the pre-clinical phase of development, with very promising results in terms of safety and efficacy against aggressive cancers (lymphomas, leukemias and pancreatic cancer) as well as for HIV Cure. The scope of this fundrasinig campaign is to complete preclinical development and move to clinica trials. Pharma potential partners have already expressed interest to support us once VS2-370 is ready to move to humans.
Regulatory Status
We have obtained EMA (European FDA equivalent) scientific advise on how to develop VS2-370
How we will use the funds raised
Aim of the present project is to complete the preclinical development of VS2-370 by:
o Concluding in vitro stability and bioavailability studies (already collected promising stability and exposure profile data in vivo)
o Performing Good Laboratory Practice (GLP) toxicology studies in rodents and non-rodents (we have already collected Maximal Tolerated Dose –MTD– and Dose Range Finding –DRF– non-GLP data in the same species)
o Writing an Investigational Medicinal Product Dossier –IMPD– essential to move into clinical trials (we have already obtained EMA scientific advices)
o Synthesizing a VS2-370 clinical batch (we presently have 1 Kg material for preclinical GLP studies)
Thank You
We have dedicated our life to control HIV and transfrom a once deadly disease into a chronic treatable one. And we succeded. It is now time to move to the next challenge: to Cure HIV. Please help us suceed again.
Updates
No updates found .
Supporters
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.
Index Score
17
Score
0
Score
1
Likes0
Partners0
Pilots0
Follows-
This campaign has ended but you can still get involved.See options below.
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.