mConnect: Universal Low Cost Home Health Hub
An inexpensive ($20) small home hub that can work with any Connected Health device (i.e. blood pressure, weight scales, etc).
Menlo Park, CA United States Diabetes Wearables Medical Device MedStartr Ventures challengeAbout our project

The problem we solve: A lot of people would like to have a permanent connection to their doctor or provider. They need to upload their vitals, get feedback, and more. Smartphones are a great tool for those who are familiar with them, but they are difficult for the elderly and less experienced users. A Home Health Hub option is desired - but there are no affordable ones on the market, existing options cost $150 or more. It also has to be universal - a patient can go to Walgreens, buy a connected product they like (i.e. any scale), and it shall just work. Alternatively, a provider or telehealth company shall be able to assemble the kit they like while keeping the cost under control.
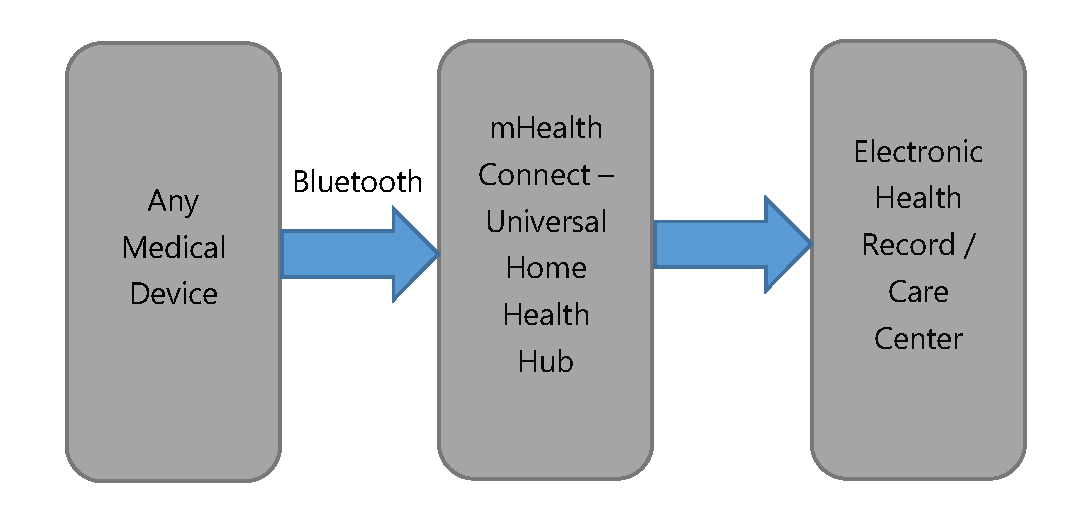
About our solution: We suggest to create an inexpensive ($20 for small orders) Home Health Hub compatible with any existing medical device or wearable. We already have more than 300 compatible devices to our smartphone platform, and it is easy enough to extend it to hub.
Progress to date:
We have a full software platform - MedM Platform - to work on the hub. We have medical device integrations, remote management, provisioning, reference cloud platform, and more. We just need to design and produce the actual box.
About Our Team

Creator: Michael Pliskin
Location: California
Bio: Engineer, Leader, Customer Advocate, Partnership Master, Startup and Corporate Veteran. Passionate about change, technologies, and changing the world. Previous experience includes startups (Comapping.com) as well as all steps as career software engineer from an intern to Technical Director.
Title: CEO
Advanced Degree(s): MS
About Team Members
Denis Khitrov
COO, MS
Biography: IT & Mobility Guru Strategist, Innovator, Problem Solver, Team Leader and Motivator. Ex. PM in SPB.COM
Title: COO
Advanced Degree(s): MS
Twitter:
@deniskhitrov
LinkedIn:
https://www.linkedin.com/in/deniskhitrov
About Our Company

MedM
Location: 1016 Middle Ave
6
Menlo Park, CA 94025
US
Founded: 2011
Website: http://www.medm.com
Twitter: @medminc
Facebook: http://facebook.com/MedMcom
Product Stage: In the Market
YTD Sales: $250,000..1M
Employees: 10-20
How We Help Patients
The product (with the appropriate service plan from MedM or others) can be sold in retail for an affordable price, and thus millions of patients can be monitored and eligible for timely healthcare advice.
How We Help Physicians
Providers can now launch cost-effective remote patient monitoring projects. No longer they need to lock themselves in into 3-year contracts - everything is available with a disposable-like pricing, full service, instant roll-out, and pay-as-you-go model
How We Help Hospitals
Hospitals can now benefit from a variety of cost-effective remote patient monitoring services on favorable terms.
How We Help Partners
Partners can use the hub both as a full product or as a hardware platform in their own projects, or just re-sell the device. The market is crying to get this done.
Challenge Mission
Market Size
According to Grand View Research market analysis, US Telehealth Market Size is more than $2B in 2018, and will be close to $3B in by 2022. A lot of patients are underserved, a lot of services are being launched, and we want our hub to power all of them.
Projected 3 Year Growth
We want to be selling 1M of our hubs per year and have 10M+ patients on our platform. This allows us to capture a decent market share and keep growing by improving the product and keep innovating.
How We Will Make Money
There are two ways to get money:
1. Sell hub as a unit to go along with our MedM Cloud RPM service and make money on service.
2. Sell hubs as unit and get a margin.
About our Competition
There are the following competitors:
- Qualcomm Life with their 2net platform
- Philips Home Telehealth
- Medtronic
Our bet is on availability, cost structure, and flexibility.
Innovation Details
Intellectual Property Summary
All our ideas are open. The exact implementation is closed, but all ideas are public domain.
Clinical Information
There are a lot of companies - Qualcomm Life, Philips, IdealLife - that are using this approach already in clinical environment, and it has been validated. We are aiming to make it more affordable and cost effective.
Regulatory Status
This whole system is a Medical Device Data System and thus is an exempt from FDA regulation. However, a 510k approval might be an option at a later stage - even though not strictly required.
How we will use the funds raised
In order to meet the price point, we need to do a completely new design of the board, the case, and the tooling. We also have to use the low-cost components, and thus spend a lot of time on debugging and integration. The preliminary plan is the following:
- $12 000 design
- $18 000 case and tooling
- $10 000 integration, debugging, and testing
- $10 000 certification (FCC, PTCRB, carriers)
Thank You
We are changing the course of telemedicine. We make it available for everyone instead of boutique clinics, we make it a consumer product and an inexpensive B2B offering. This hub with its 10x cost reduction will be a disruption for the telehealth industry.
Updates
No updates found .
Supporters
-
11/30/2017 - Liked the project., Master’s Degree in Business Administration from Seton Hall University
10/06/2017 - Liked the project.
03/16/2017 - Followed the project.
01/23/2017 - Followed the project.
01/23/2017 - Interested in a partnership with the project.
12/09/2016 - Liked the project.
12/07/2016 - Followed the project.
12/05/2016 - Interested in helping your project as a mentor or team member.
12/05/2016 - Followed the project.
12/05/2016 - Followed the project.
11/30/2016 - Liked the project.
11/30/2016 - Followed the project.
11/29/2016 - Liked the project.
11/24/2016 - Liked the project.
11/24/2016 - Followed the project.
11/24/2016 - Liked the project.
11/24/2016 - Liked the project.
11/24/2016 - Liked the project. , MScPT
, MScPT
11/23/2016 - Followed the project.
11/23/2016 - Liked the project.
11/23/2016 - Liked the project.
11/23/2016 - Liked the project.
11/22/2016 - Followed the project.
11/21/2016 - Backed the project for $20
11/21/2016 - Liked the project.
11/21/2016 - Liked the project. Instant Feedback
Instant Feedback
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.
62Medstartr
Index Score37
Interest
Score5
Adoption
Score16
Likes1
Partners0
Pilots10
Follows-
This campaign has ended but you can still get involved.See options below.
$20 pledged of $50,000 goal
Instant Feedback
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.