IHC Process Record Slide (PRS): Ultimate Elimination of False Negative IHC Staining Results
by Denny Lam
The first IHC device to add quality control monitoring in accordance with WHO requirements using on-slide quality control materials and calibrators
Hong Kong, Hong Kong DiagnosticsAbout our project
The problem we solve: To this day, laboratories around the world have been unable to process patient tissue samples with co-resident control materials on each single slide in accordance with the World Health Organization (WHO)'s strict requirement. (See Quality Control in Laboratory Systems) In addition, laboratories have never had access to a calibrating system that can track and improve the IHC process.

About our solution:
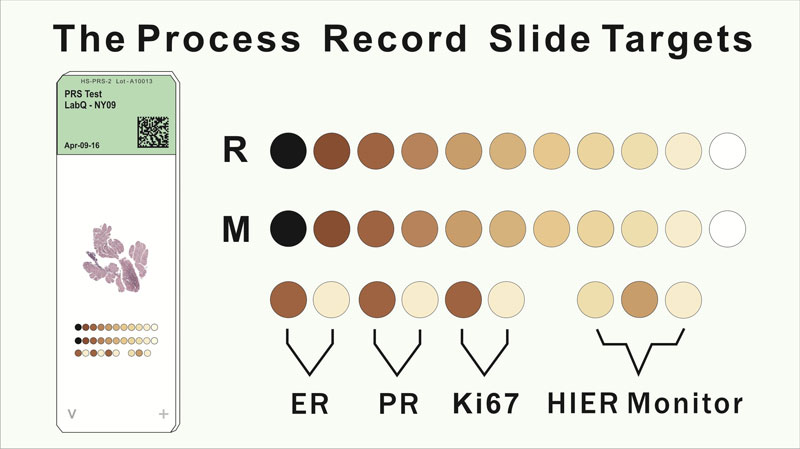
Process Record Slide is an innovative solution that adds quality control monitoring for the patient tissue section on each glass slide. It also serves as a slide by slide calibrator.
Features and Uses:
- IHC Staining - Get an effective record of the IHC staining experience
- Quality Control Evaluation - Target data provides objective QC evaluation of the processing experience
-
Concentration Ruler - Target data forms the basis of an antigen concentration ruler
-
Pre-Screening - The ruler provides a digital imaging baseline for pre-screening
-
Imaging Adjustment - The ruler supports imaging adjustment so the viewing Pathologist can make the best diagnostic interpretation
-
2nd Opinion and Remote Diagnostic Opportunity - 2nd opinion is possible when the observer can see the processing record and know what was done to the PRS and tissue section

Progress to date:
To date, a prototype has been developed and tested with success. It will be manufactured and available for purchase November-December 2017. The PRS has been listed with the FDA Class 1 as a slide by slide calibrator.
The College of Pathology has long sought a product like our PRS because they realize there is a high rate of failure in IHC staining which leads to misdiagnosis.
A recent paper, An Audit of Failed Immunohistochemical Slides in a Clinical Laboratory: The Role of On-Slide Controls, found that the failure rate of IHC staining can be as high as 9%. (Department of Pathology, Laboratory Medicine Program, University Health Network †Department of Laboratory Medicine and Pathobiology, Faculty of Medicine, University of Toronto, Toronto, ON, Canada Keck School of Medicine, University of Southern California, Los Angeles, CA.) The paper concludes that in the era of automated IHC staining platforms, on-slide controls allow for the proper identification of IHC slides that should be failed by the IHC laboratory. For this reason, on-slide controls represent a powerful tool for preventing the reporting of false-negative/false-positive tests.
We will be exhibiting our PRS solution at International conferences for pathology this year, including:
- US Bio 2017 - San Diego - 19-22 Jun
- 13th Pathology & Molecular Diagnosis Conference - San Diego - 26-27 Jun
- 8th European Immunology Conference - Madrid - 29-1 July
- 13th European Pathology Congress - Milan - 2-3 Aug
- Digital Pathology USA – Philadelphia 10th – 11th July
- Digital Pathology Asia – Guangzhou 16th- 17th September
- Digital Pathology Europe – London 30th November – 1st December
The image above shows part of our design for the machine producing our PRS. All drawings are now completed. The machines are under construction and we expect them to be finished by September 2017.
About Our Team
 Jee Shum, Chief of R&D
Jee Shum, Chief of R&D
Mr. Shum has more than 40 years experience in product manufacturing, which has included stadium class light message boards, computers, pick & place assembly robotics, and laser engraving. Mr. Shum has owned and managed a number of successful companies both in the USA and foreign, with the most recent being American PC. Additionally, Mr. Shum is experienced in negotiating and managing manufacturing in China.
R&D efforts have included:
- Heat driven 2-phase heat exchanger, film boiler & condenser design
- IHC process control slide: polymer, surface, & biochemistry and automation
 Frederick Husher, Chief of R&D
Frederick Husher, Chief of R&D
Mr. Husher has more than 40 years experience in R&D, product development, and business development. Mr. Husher has worked for Tektronix, University of New Mexico, Megiddo, US Military Joint Services, Amtech, Gtech, Beckman Coulter, Veracel, Barfield EADS, JAF Development Corp, and Crane Cams in various Electrical Engineering and Management positions.
R&D efforts have included:
- IHC process control slide: polymer, surface, & biochemistry; automation,; and analysis
- Ultrasonic based analysis of blood to determine ESR, ZSR, and viscosity during aspiration
- RF linked epilepsy brainwave monitoring for evaluating drug dosages on patients
- Solid-state RF oscillator conductivity detector functional to twice Nyquest noise floor used for white blood cell measurement
- Automated blood smear maker
- Automated PAP smear sample cleaning and slide smear maker
About Our Team

Creator: Denny Lam
Education: St. Francis Xavier's College
Bio: Investor in innovative healthcare products.
Hospital Affiliation: NIL
Title: Managing Director
Advanced Degree(s): NIL
About Our Company

Process Record Slide Limited
Location: Unit D, 3/F., Freder Centre
Mok Cheong Street, Tokwawan
Hong Kong 999077
HK
Founded: 2006
Website: http://www.ihc-prs.com
Twitter: @IHC_PRS
Facebook: https://www.facebook.com/Process-Record-Slide-IHC-Biopsy-1106685979445980/
Other link: https://www.linkedin.com/company-beta/13326404
Product Stage: Prototype/MVP
YTD Sales: 1M...5M
Employees: 3-5
How We Help Physicians
The Process Record Slide (PRS) contains an array of immunohistochemical (IHC) reactive targets that are co-resident with an applied patient tissue section. Both the target array and patient tissue section go through the full IHC processing experience together. The target array's reactivity to one or more of the staining reagents records the cumulative experience of the co-resident tissue section. Human origin materials are not used as part of the target arrays. Thus, each PRS contains its own unique IHC processing experience record for the co-resident tissue section. Slide-to-slide analysis of the PRS array data can be used in quality control of the IHC process using statistical variance tracking. The PRS target array provides digital imaging with calibrating targets that uniquely expresses the staining experience of the co-resident tissue section.
With our PRS, you will be able to:
- Provide a record of the processing experience that target and the tissue section experienced. The target data can be used to verify that the processing and reagents are functioning within their tolerance limits as defined in the laboratory’s process control documents.
- Record the IHC staining experience and identify process or reagent deficiencies that may occur.
- Change the subjective diagnostic interpretation into objective interpretation because the efficacy of the IHC processing is known to be valid via the PRS target results.
- Validate and support corroborative 2nd opinion and tele-diagnostics within even remote communities across the globe.
Innovation Details
Intellectual Property Summary
We have filed six provisional patents application and one trademark by Norton Rose Fullbright in NY.
Patent Link
Not available
Clinical Information
The goal of our campaign is to raise funds for further research and manufacturing. In addition, we also need a scalable demand to reduce the cost of production so that the cost of each PRS becomes affordable for every pathology lab. We aim to reach 2000 pathology labs who will each use 5 - 10 pcs of our PRS as a part of a pre-launch trial.
To the best of our knowledge, FDA has not granted approval to any IHC and H&E scanners (Leica, Ventana - Roche) because there has never been a product like PRS that can provide on slide control and on-slide calibration. Failing to provide this service can lead to inaccurate staining results.
A recent paper discovered that up to 9 slides out of 100 are stained incorrectly, which is an unacceptably high failing rate for a clinical test. The authors emphasized that on slide control for every single IHC staining process ought to be required. A control slide can be produced manually in a lab but is economically unviable due to the cost and time required to do so.
The introduction of our PRS will provide quality control for every IHC staining experience.
Regulatory Status
FDA registration has been completed:
Medical: FDA Class 1 listing, number D296210 - as slide by slide calibrator
How we will use the funds raised
-
Manufacturing the Process Record Slide
-
Further research on other primary IHC targets on slide
Thank You
A medical device can take years to bring to the market. We believe PRS is a game-changing solution that we want to put in the hands of pathologists and lab technicians as early as November 2017. We can’t do this without your help and investment. If you think our PRS solution could help your clinical laboratory, we urge you to contribute to our campaign by selecting one of the rewards listed above.

(Displayed Left to Right) PRS for IHC, H&E Slide, Pap Smear Slide
Updates
No updates found .
Supporters
-
, Master’s Degree in Business Administration from Seton Hall University
10/06/2017 - Liked the project.
08/17/2017 - Liked the project.
07/18/2017 - Liked the project. , NIL
, NIL
07/15/2017 - Followed the project.
07/12/2017 - Liked the project.
07/07/2017 - Liked the project. , NIL
, NIL
07/06/2017 - Liked the project.
07/05/2017 - Followed the project.
06/30/2017 - Interested in trying the project. Instant Feedback
Instant Feedback
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.
14Medstartr
Index Score9
Interest
Score1
Adoption
Score6
Likes0
Partners0
Pilots0
Follows-
This campaign has ended but you can still get involved.See options below.
$ 20,000 goal
Instant Feedback
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.


