Ideal Medical Technologies: Artificial Pancreas to lower ICU mortality rates
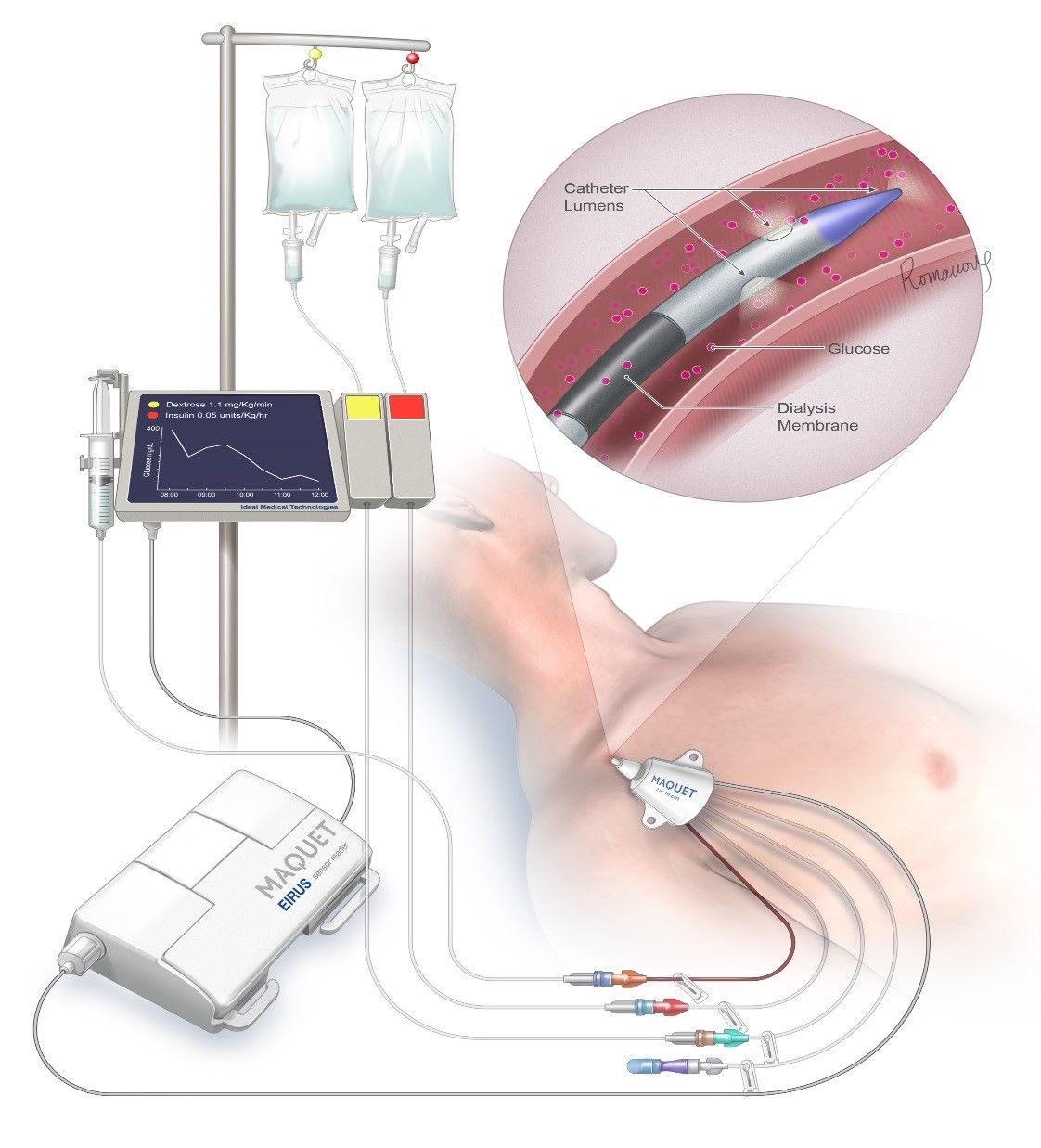
Our FUSION system will autonomously control the glucose levels of ICU patients which will lower their mortality rates by >30%, reduce their cost of care by >$1,500 and make nurses more efficient
Asheville, NC United States Hospital Solutions Medical DeviceAbout our project
The problem we solve: Ineffective glucose control in the ICU setting leads to high rates of hyperglycemia, hypoglycemia and increased glucose variability. This altered glucose state adversely affects the white blood cells and sets off the bodies inflammatory cascades, which leads to increased infections and multiple organ failure. Glucose control is currently done via an open loop method whereby the nurse manually checks the glucose level, manually enters this into an insulin dosing calculator, and manually adjusts the intravenous rate of insulin infusing into the patient. This method is not very effective and is very time consuming. There are currently no approved artificial pancreas systems available for use in the hospital setting in either the U.S. or European Union.

About our solution: Our FUSION artificial pancreas system is a fully autonomous closed loop glucose control system that will only take 10 minutes to set up and will provide far more effective glucose control than can currently be achieved by the current open loop method. In addition, it will free up the nurses to spend more time in direct patient care. This will be very important during the COVID-19 pandemic as there will be a critical shortage of ICU nurses.

Progress to date:
We already have a working prototype and have finished all of our preclinical testing, thus at this time we are ready to begin clinical studies. We had planned on pursuing a step wise approach to our clinical studies with plans to bring our FUSION sytem to market by Q1 2022. However, given with the current COVID-19 pandemic causing a daily loss of life now approaching one thousand people, our new plan is to accelerate testing of a beta version of our FUSION system by June 2020. We should be able to accomplish this as our glucose control software and operating system have already been fully developed and we plan to use off the shelf continuous glucose monitors (e.g., Dexcom G6 or EIRUS) and intravenous pumps. Once we prove the safety of the beta version of our FUSION system in an early feasibility study in ICU patients, we will petition the FDA for an Emergency Use Authorization given its potential to lower ICU mortality rates during the pandemic.
About Our Team

Creator: Leon DeJournett
Location: North Carolina
Education: Chicago Medical School-Rosalind Franklin
Bio: Dr. Leon DeJournett has more than thirty years of experience working in the intensive care unit (ICU) setting. He first became interested in glucose control in the ICU in 2007. After performing an extensive review of the literature in this area, he realized that the control methods in use at that time would not be able to provide safe and effective glucose control as they were not capable of keeping up with the highly dynamic blood glucose levels seen in ICU patients. By combining his ICU experience, a deep understanding of how the native system works, and computer coding skills, he was able to write algorithms that mimic the workings of the native pancreas (insulin release) and liver (glucose release), as it pertains to glucose control. These algorithms exhibit biomimicry and form an expert based rule system, which is considered a form of artificial intelligence (AI). Dr. DeJournett has several peer reviewed publications related to the testing of this AI based glucose control software, including a recent publication that showed superior glucose control when it was tested head up against an ICU physician. In 2014 he formed the Ideal Medical Technologies corporation and subsequently led its development efforts of a closed loop glucose control system (artificial pancreas), which has been given the name FUSION. He led the effort to obtain Breakthrough Medical Device designation status for the FUSION system, which was granted by the FDA in 2019 as an acknowledgement that the FUSION system has the potential to decrease the mortality rates of ICU patients. There are currently no approved artificial pancreas systems available for use in the hospital or ICU setting in either the U.S. or EU. Dr. DeJournett received his medical training at Stanford University Medical Center.
Hospital Affiliation: Ideal Medical Technologies
Title: Founder, Chief Medical Officer
Advanced Degree(s): MD
About Team Members
Alan Jernigan
CEO, MBA
Biography: Alan Jernigan has more than 25 years of progressively responsible experience directing as many as 300 employees in companies with revenues in excess of $3 billion. Alan has led these companies through start-up, survival, turnaround, growth modes and successful exit.
Alan has spent 20 years as a Senior Executive in a variety of medical, pharmaceutical and biotech industries both on the human and veterinary side. His understanding of both domestic and international point of care sales/marketing/distribution channels & markets encompasses physician offices, hospitals, military and retail clinic sales, wholesale, OEM, with both direct and indirect sales forces. He has personally been involved with successfully launching and growing over 50 medical device, point of care & IVD diagnostic products.
Among his significant previous positions, Alan has served as CEO of three different start-ups which had successful exits and returned significant value for its shareholders. He has also held several VP sales & marketing for large and start-up companies such as Abbott, Roche, ThromboVision Inc., and IGEN.
Alan Jernigan holds a Bachelor of Science degree from Oklahoma State University and a Master of Business Administration (MBA) degree from Oklahoma City University.
Title: CEO
Advanced Degree(s): MBA
LinkedIn:
https://www.linkedin.com/in/alan-j-123b0038/
Jeremy DeJournett
Chief Technical Officer, BS, Electrical Engineering
Biography: Jeremy DeJournett serves as the technical lead and acting Chief Technology Officer for Ideal Medical Technologies. He graduated from the North Carolina School of Science and Mathematics in May 2012, and completed a Bachelor of Science in Electrical Engineering from the University of Illinois at Urbana-Champaign in May 2018.
While in college, he worked with a NASA funded CubeSat lab through the Satellite Development Organization, and successfully delivered the CubeSail satellite to Rocket Lab in March of 2018. He was responsible for delivering high level technical reports to principal investigators, as well as translating requirements into technical specifications for the embedded software development team that he led.
In parallel to this work, he was key to the development of Ideal Medical Technologies’ technical stack. During the fall of 2015, he completed the first major revision of the FUSION software, which was used to run a pre-clinical trial at Wake Forest in December 2015. The following summer, he developed the FUSION simulator, which was used to perform large scale combinatorial analysis of the FUSION software, and to demonstrate the efficacy of the control methodologies in both typical and difficult clinical and non-clinical scenarios.
Through winter of 2016 and spring of 2017, he further extended the FUSION simulator to support other glucose controllers that have seen clinical use, which was used to publish a comparative study of the FUSION controller. He successfully integrated the FUSION controller with the EIRUS CE marked continuous glucose monitor from Getinge, AB, and tested this working prototype in pre-clinical trials in October 2018. This work included fault tolerance analysis, drug delivery verification, and user interface improvements. He has produced all of the system design and technical documentation required for the Investigational Device Exemption application to the FDA.
Title: Chief Technical Officer
Advanced Degree(s): BS, Electrical Engineering
LinkedIn:
https://www.linkedin.com/in/jeremy-dejournett-4b1340123/
About Our Company
Ideal Medical Technologies
Location: 18 N Kensington Rd
Asheville, NC 28804
US
Founded: 2014
Website: http://www.idealmedtech.com
Product Stage: Prototype/MVP
Employees: 3-5
How We Help Patients
If you or someone you care for has ever been admitted to an intensive care unit (ICU), you can understand how stressful this situation can be for a persons body. What you may not have realized is how important it is to keep the blood glucose level of critically ill patients locked into a zone, such as 100-140 mg/dL, while simultaneously avoiding hypoglycemia and extremes of glucose fluctuation (variability). ICU nurses sometimes have to care for up to 3 patients at a time, and with the current COVID-19 pandemic this number may rise to more than 3 patients per ICU nurse as there will end up being a shortage of ICU nurses. The only way to lock in the glucose level of an ICU patient is to use an artificial pancreas system. The ICU nurse cannot possibly maintain tight control of their patients glucose level by checking it every 1-4 hours, as the glucose level of an ICU patient can fluctuate at a rate of up to 3 mg/dL/minute! Thiis means that it can change by more than 100 mg/dL over the course of one hour. Only by following the blood glucose level with a continuous glucose monitor that gives new glucose readings at least every 5 minutes, and adjusting the rates of insulin and glucose (dextrose) flowing into the critically ill patient, can you hope to keep steady a critically ill patients glucose level. Our FUSION system uses both insulin and glucose infusions to maintain control of the glucose level, and makes adjustments every 5/10 minutes to its infusion rates. Altered glucose metabolism in ICU patients is a silent but very real killer! We believe our FUSION system will work just as well on diabetic patients in a non-ICU setting, as they also suffer from poor glucose control using the current control method of subcutaneous insulin injections 2-3 times/day. If you have diabetes and have ever been admitted to the hospital, you will understand how difficult it is for your nurses/doctors to achieve safe and effective glucose control. Only through mimicking how the bodies natural glucose control system works can we hope to achieve safe and effective glucose control for very sick hospitalized patients. This is how the FUSION system works, through biomimicry.
How We Help Physicians
If you provide care for patients in the hospital setting, you know how difficult it can be to achieve safe and effective control of a patients blood glucose level. With the current open loop methods, the bedside nurse must manually check the patients blood glucose level, manually enter this value into an insulin dosing calculator or refer to the insulin order set, then manually administer the insulin (e.g., adjusts IV pump for ICU patients or gives subcutaneous injection for diabetic patients in a non-ICU setting). This can lead to human error at all three steps. The current open loop method is time consuming and frankly not very effective. Despite all of the evidence supporting improved patient outcomes with safe and effective glucose control in both the ICU and non-ICU setting, we still do not have any approved artificial pancreas systems available for use in the hospital setting, let alone one that can actually achieve a high level of control. Imagine if glucose control could be as simple as the physician ordering what glucose range they want their patient to be in and our FUSION system locks the patient into this range with no hypoglycemia and minimal glucose variability. The FUSION system only takes 10 minutes to set up, thus the nurse will be freed up to spend more time on direct patient care, which should also serve to improve both patient outcomes due to the increased time the nurse can spend in direct observation of their patient, and patient satisfaction as they will be seeing more of their nurse.
How We Help Hospitals
If you are a hospital CEO, CFO or CMO you need to realize how big a problem inpatient glucose control is. While ICU beds may account for less than 10% of your total bed capacity, ICU patients account for almost 15% of your total patient costs. Caring for ICU patients is expensive! In addition, diabetic patients make up 25-30% of your patient volume admitted into a non-ICU setting, and as they tend to spend almost a week in the hospital, they account for more than 30% of your patient costs when looking at just the non-ICU bed environment. Your reimbursement for Medicare and Medicaid patients (typically >50% of your patients) is based on the patients diagnostic related groups (DRG's). This is a fixed reimbursement, thus anything you can do to decrease the cost of caring for these patients will improve your profit margin. As you most likely operate with a profit margin of 2-5%, even small improvements will help you maintain your hospital as an ongoing business concern (true for both nonprofit and for profit hospitals). Studies have shown that ICU patients with improved glucose control achieve cost savings in the range of $1,500-$4,000 per patient (Krinsley, Chest, 2006; Sadhu, Diabetes Care, 2008). Studies have also shown cost savings of $50,000 per patient in the group of abdominal surgery patients who did not experience any hyperglycemia in the post operative period (Buehler, J of Diabetes Complications, 2015). This was true for both diabetic and non-diabetic patients, thus the benefits of safe and effective glucose control may extend as well into the non-diabetic patient population undergoing major surgeries. This abdominal surgery study was done on non-ICU patients. With an average ICU length of stay of 4 days, you should be able to use the FUSION system 90 times per year. If the FUSION system produces net cost savings of $1,500 per patient, this will lead to annual cost savings of $135,000 for each FUSION system in use. The FUSION artificial pancreas system will be a complete win for your hospital - better patient outcomes, improved nursing efficiency, one less patient issue for physicians to stress about, improved hospital financial performance.
How We Help Partners
Our FUSION system will be of major benefit to the U.S. government and insurance companies, especially with the current COVID-19 pandemic. ICU care is very costly, with the average ICU patients care costing $25,000 ($150 billion/year for 6 million patients/year = $25,000/patient). For more complicated ICU patients who spend more than 4 days in the ICU the cost of their ICU care can easily exceed $100,000. Both the U.S. government and insurance companies are going to end up paying for the care of the upcoming wave of COVID-19 ICU patients, and our FUSION system has the potential to decrease the total cost of this care. In addition, once the COVID-19 pandemic has passed, our FUSION system will continue to decrease the cost of caring for ICU patients and diabetic patients in a non-ICU setting in perpetuity. Finally, because our FUSION system works autonomously and requires very little expertise to run, it will allow for safe and effective glucose control to be achieved for hospitalized patients throughout the world.
Innovation Details
Intellectual Property Summary
Ideal Medical Technologies has been issued patent protection for its complete glucose control system in both the U.S. and European Union. The U.S. patent number is 8,956,321. It was issued on February 17, 2015 and is good through 2031. The European patent number is 2197521B1. It was issued on April 13, 2016 and is good through 2028.
Patent Link
https://patents.google.com/patent/US8956321B2/en
Clinical Information
Over the past 20 years there have been multiple studies performed in the ICU setting documenting improved patient outcomes with tight glucose control. Here are a few well known examples:
1. Hypoglycemia - In a study done by Egi (Mayo Clin Proc, 2010) on 4,946 ICU patients, it was shown that patients who did not experience any glucose levels lower than 81 mg/dL had a 46% lower mortality rate.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2843109/pdf/mayoclinproc_85_3_003.pdf
2. Mean glucose and glucose variability - In a study done by Krinsley (J Diabetes Sci Tech, 2009) on 3,142 non-diabetic ICU patients, it was shown that patients with a mean glucose level of 100-119 mg/dL and a glucose variability (CV = standard deviation/mean glucose) of less than 15%, had a 300% reduction in their mortality rates compared to patients with higher mean glucose levels and higher variability.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2787029/pdf/dst-03-1292.pdf
3. Time in range 70-140 mg/dL - In a second study done by Krinsley (Critical Care, 2015) on 2,550 non-diabetic ICU patients, it was shown that patients who achieved a lower time in the physician prescribed range of 70-140 mg/dL had a 86% increase in their mortality rate compared to patients who achieved a high time in the range of 70-140 mg/dL.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4446958/pdf/13054_2015_Article_908.pdf
4. Low versus high glucose control range - In a Japanese study done by Okabayashi (Diabetes Care, 2014) on 447 surgical ICU patients, it was shown that controlling patients to a lower range of 80-110 mg/dL versus a higher range of140-180 mg/dL led to a 58% reduction in their post-operative surgical wound infection rate and a 21% reduction in their hospital length of stay.
https://care.diabetesjournals.org/content/37/6/1516.full-text.pdf
5. Cost reduction - In a study done by Cardona (J of Diabetes and its Complications, 2017) on 288 coronary artery bypass graft patients, the group of patients controlled to a lower glucose range in the post-operative period had cost savings of $3,654/patient..
https://www.sciencedirect.com/science/article/abs/pii/S1056872716308984?via%3Dihub
6. Cost reduction - In a study done by Van den Berghe (Crit Care Med, 2006) on 1,548 mechanically ventilated ICU patients, a cost reduction of $3,300 per patient was shown in the group of patients controlled to the lower glucose range of 80-110 mg/dL.
https://www.ncbi.nlm.nih.gov/pubmed/16521256
7. Nursing time - In a study done by Aragon (Amer J of Crit Care, 2006) on the time ICU nurses spend on tight glucose control efforts, it was shown that ICU nurses will spend up to 2 hours per patient per day attempting to control blood glucose levels using the current open loop method.
https://www.ncbi.nlm.nih.gov/pubmed/168230148.
8. Simulation study of FUSION systems AI-based glucose control software - In a simulation study done by DeJournett, L (J of Diabetes Sci and Technol, 2016) it was shown that our FUSION systems AI based glucose control software, in simulated ICU patients undergoing time variant stresses (e.g., variable insulin sensitivty) during the course of a 5 day simulation, was able to achieve the following results: 1) percent time in range 70-140 mg/dl of 97.8%, 2) percent time in range < 70 mg/dL of 0.09%, glucose variability (CV) of 11.1%. Simulations are accepted by the FDA as a valid method to assess the potential functionality of glucose control software.
https://europepmc.org/backend/ptpmcrender.fcgi?accid=PMC5094333&blobtype=pdf
9. Comparative simulation study of FUSION systems AI-based glucose control software - In a comparative simulation study done by DeJournett, J (J of Diabetes Sci and Technol, 2017) it was shown that when our FUSION systems AI-based glucose control software was compared to other currently in use insulin dosing calculators designed for use in the ICU setting, that our overall glucose control score was 75% better than the next best control system. Our FUSION systems glucose control software experienced no hypolgycemia (<70 mg/dL), had a percent time in range 70-140 mg/dL of 96.7% and a CV of 9.9%.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5951048/pdf/10.1177_1932296817711297.pdf
10. Comparative animal study of FUSION system prototype versus an experienced ICU physician - In a comparative study done by DeJournett, J (J Clin Monitoring and Computing, 2020), it was shown that when the FUSION system was compared head up to an experienced ICU physician in an animal study involving unannounced (to the FUSION system) overdoses of insulin and glucose, that the FUSION system outperformed the ICU physician in every hypoglycemic measurement, even though the FUSION system was tasked with controlling to a tighter range than the ICU physician (4.4-6.6 mmol/L vs 4.4-9 mmol/L). Controlling to a tighter control range should have made the FUSION system more prone to hypoglycemia, however, given its superior control method the FUSION system easily outperformed the ICU physician.
https://link.springer.com/article/10.1007%2Fs10877-020-00474-2
The FDA is aware of our progress on bringing our FUSION closed loop glucose control system to market. During the course of our pre-submission meetings with the FDA in 2019 we mapped out a series of clinical studies that would be needed to bring our FUSION system to market by Q1 2022. We had been planning on performing our first clinical study this summer, however, given the current COVID-19 pandemic with its extremely high mortality rate, we plan on accelerating our plan to bring our FUSION system to market as we believe it may be able to lower COVID-19 mortality rates by at least 20%. We already have a fully functional prototype that works with different types of continuous glucose monitors (e.g., Dexcom G6 and EIRUS), thus we believe we can work with an already identified contract manufacturer to create a beta version of our FUSION device that will be fully functional and meet FDA requirements for an early feasibilty study by June, 2020. We will seek IRB approval for our early feasibility study around the same time we seek an investigational device exemption (IDE) from the FDA. The clinical protocol for this early feasibility study has already been written.
Regulatory Status
In 2019 the FDA designated our FUSION device as a Breakthrough Medical Device given its potential to lower ICU mortality rates, if it performs as well in the ICU setting as it has in both our simulation and animal studies. The FDA also acknowledges the need for an artificial pancreas intended for use in the hospital setting, as none currently exists. We have already written our IDE application for our first in human early feasibility study using our prototype, however at this time, given the COVID-19 pandemic, we plan on working with our contract manufacturer to assemble a beta unit of our commercial grade device, seek an IDE from the FDA for testing this beta unit in the ICU setting, then seek Emergency Use Authorization from the FDA to quickly bring it to market, assuming the beta unit performs well in our early feasibility ICU study. This whole process should only take 3-4 months as we will mostly be using off the shelf components - continuous glucose monitor and intravenous pumps. Our glucose control software and operating system has already been fully developed. During our pre-submission meetings with the FDA in 2019 they determined that we will be eligible to bring our FUSION artificial pancreas system to market as a class II medical device under the De Novo pathway (glucose control software).
How we will use the funds raised
The funds will be used to transfer our glucose control software from its current laptop environment to a computer tablet that will serve as the user interface for the system. The funds will also be used to pay the contract manufacturer for assembling the final device components (tablet, Intravenous pumps, battery backup) and mounting them on an IV pole, and developing the devices design history file for our submission to the FDA. The funds will also be used to initiate work on a more finished version of the the FUSION system, which we expect we can have ready within 12 months. The contract manufacturer will be capable of manufacturing and distributing the device. Some of the funds will also be used to pay for a contract research organization to assist with our clinical trial, and a regulatory consultant to assist with our FDA filing.
Thank You
Safe and effective glucose control is extremely important for all ICU patients, and all diabetic patients admitted to a non-ICU setting. The current method of glucose control is an open loop method, whereby the nurse manually checks the patients blood glucose level, manually enters this value into an insulin dosing calculator, and then manually adjusts the rate of intravenous insulin (ICU patient) or administers the subcutaneous dose of insulin (diabetic patient in a non-ICU setting). This open loop method does not produce safe and effective glucose control, and is very time consuming for the nurses. Our FUSION system should be able to save >100K lives and decrease our nations healthcare costs by more than 5 billion dollars - we currently spend 220 billion dollars per year in the care of these patients.
The COVID-19 pandemic has changed the need for the FUSION sytem from urgent to emergent - we need your help in getting this device to market! If the COVID-19 pandemic ends up infecting one billion people with a 2% mortality rate, 20 million people will die. If our FUSION device can lower this mortality rate by 20%, together we may be responsible for saving more than 4 million lives!! Lets work together to fight this pandemic!!!
Updates
No updates found .
Supporters
There are not supporters yet.
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.
0
Score
0
Score
0
Likes0
Partners0
Pilots0
Follows-
This campaign has ended but you can still get involved.See options below.
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.

