SmartSteward: Helping save lives in Nursing Homes
by Guy LaTorre
Real-time Infection surveillance & alert and clinical decision support for busy nursing home to detect deadly outbreaks and reduce infections by controlling antibiotic resistance
Gainesville, FL United States Infectious Disease 2021 Vision challengeAbout our project

The problem we solve: The global COVID-19 pandemic has been one of the deadliest viral outbreaks in the U.S. in over 100 years. The virus has been particularly deadly in Nursing Homes, one of the hardest hit healthcare settings, with residents and staff members accounting for almost 40% of the 200,000 coronavirus-related deaths in the U.S. The current CDC recommendations are for a strong infection prevention and control program, including performing infection surveillance, which is critical to protect both residents and healthcare personnel. COVID-19 is currently the biggest threat to Nursing Home residents, but they were already suffering – from the multi-drug resistant organisms (MDRO) epidemic. According to the CDC, more than 2.8 million antibiotic-resistant infections occur in the U.S. each year, and more than 35,000 people die as a result. Among all factors contributing to antibiotic resistance and the exponential growth of deadly MDRO infections is the overuse and misuse of antibiotics.
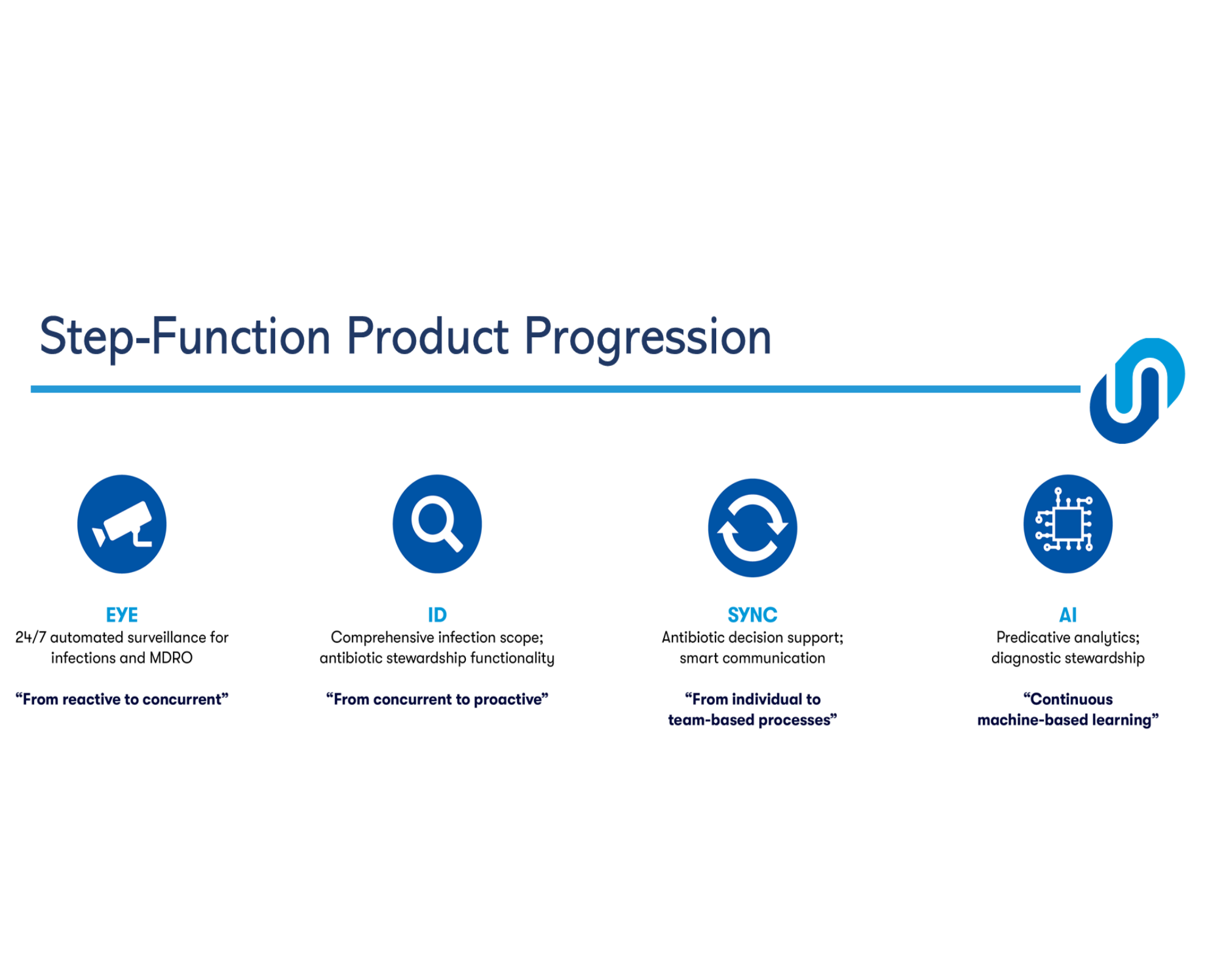
About our solution: SmartSteward Inc. has created a modular system that starts with the SmartSteward™ EYE, an Infection Surveillance & Alert System that fills the unmet need for tools for early detection of COVID, Influenza and MDROs in Nursing Homes in real-time. The SmartSteward EYE can immediately help address COVID-19 in Nursing Homes today, and will be an important foundation to help facilities automate the infection control process post-pandemic. Facilities can begin by implementing this surveillance module, then add additional modules that optimize antibiotic prescribing and reduce facility-wide antibiotic resistance.
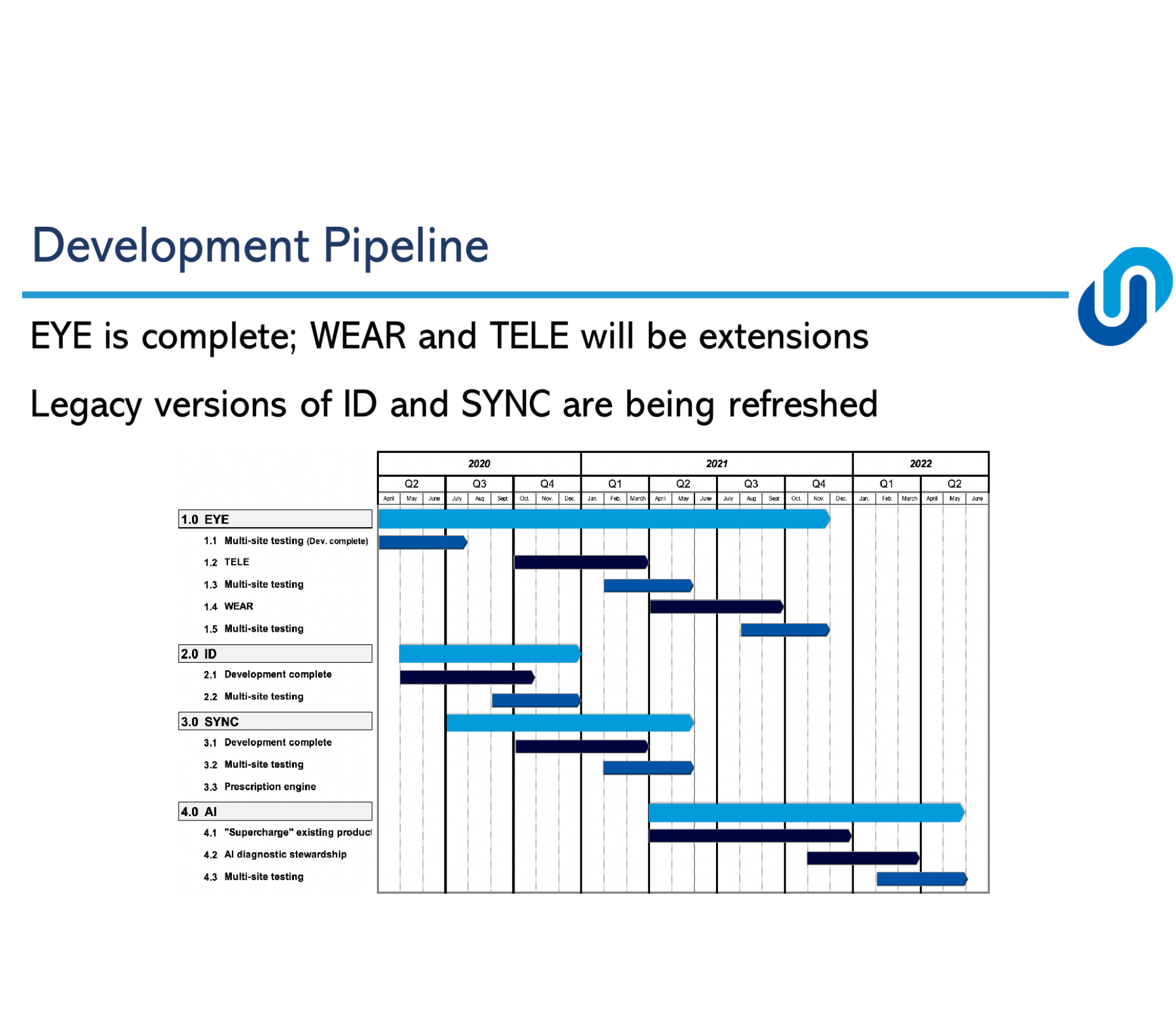
Progress to date:
We have achieved the following milestones this past year including:
- Completing the development of our first module the SmartSteward EYE Surveillance & Alert System
- Initiated 3 pilot studies to validate this new product in nursing homes with early positive results:
- Accurately Identified 79% of the symptomatic residents who later tested positive
- Detected potential COVID cases in the facility 7 to 14 days in advance of the first diagnosis
- Unnecessary use of antibiotic reduced 30%, measured in days of therapy per 1,000 patient days
- Reduction of facility acquired infection rate from 6.56 to .87 per 1,000 patient days
- Unplanned readmissions to hospitals declined by 25%
- Initiated discussions with three potential partners in the LTC space
- Signed 2 channel distribution partner agreements
- Signed our first customer
About Our Team

Creator: Guy LaTorre
Location: Florida
Education: University of Florida
Bio: Guy is the Company’s CEO & Chairman of the Board. He has over 25 years’ experience in medical device development and commercialization. He has served in numerous executive management positions in the regenerative medicine, dental and IVD spaces, and has had successful exits with previous companies. Guy has expertise developing solid global partnerships in multiple sectors.
Title: CEO
About Team Members
Jeffrey Goodman
CCO, B.S., MBA
Biography: Jeff, the Company’s Chief Commercial Officer, has extensive business development and entrepreneurial experience within the medical device industry and has led sales and marketing efforts for market leaders and innovators. He is the founder and CEO of a successful contract sales consultancy.
Title: CCO
Advanced Degree(s): B.S., MBA
LinkedIn:
https://www.linkedin.com/in/jeffrey-goodman-6651033a/
Robert Yancey
Medical Director, MD
Biography: Bob, SmartSteward’s founder and Chief Medical Officer, is an Infectious Disease Specialist and Epidemiologist with more than 20 years’ experience in antibiotic stewardship and infection control. He has designed and successfully implemented four different hospital-based Antibiotic Stewardship programs. Bob has assembled a dynamic team of clinical, software development and business commercialization experts.
Title: Medical Director
Advanced Degree(s): MD
LinkedIn:
https://www.linkedin.com/in/bobyancey/
Sam Borgert
Consultant ID Pharamcist, PhD
Biography: Sam is an experienced clinical pharmacist with expertise in antimicrobial stewardship. In addition to being an exceptional clinician, he has over thirty years of patient care, consulting, pharmacy administration, and change management experience.
Title: Consultant ID Pharamcist
Advanced Degree(s): PhD
LinkedIn:
https://www.linkedin.com/in/sam-borgert-284a3733/
About Our Company

SmartSteward Inc.
Location: 747 SW 2nd Ave
#43
Gainesville, FL 32601
US
Founded: 2014
Website: https://smartsteward.co/
Facebook: https://www.facebook.com/smartstewardco/
Product Stage: Ready
Employees: 5-10
How We Help Patients
Respiratory infections like COVID and Influenza as well as antibiotic resistant bacteria like MRSA are responsible for the deaths of thousands of nursing home patients in the U.S. every year. It is well established that infection control and antibiotic stewardship programs can significantly reduce the spread and severity of infections resulting in reduced complications and better patient outcomesHow We Help Physicians
Nursing home doctors are often off site as they work for multiple facilities. When nurses identify a patient with a potential infection, they usually communicate with the doctor via phone call or text often sharing information in a non HIPAA compliant manner. SmartSteward enables better communication and data sharing at the point of care giving nurses and doctors real-time information to make better treatment decisions. SmartSteward helps nurses evaluate potential infections and share patient data with doctors more efficiently. Doctors obtain access to critical patient information during the prescribing process and helps guide them to make the best treatment decisions for their patients through optimizing antibiotic prescribing.How We Help Hospitals
Nursing homes get significant fines from CMS if they have to many infections and readmissions into the hospital and can lose up to 2% of their CMS revenue and damage their 5 star rating. SmartSteward has been shown in our pilot studies to reduces infection rates and readmissions to the hospital saving both time and money for the facilityHow We Help Partners
The overall direct cost of Health Acquired Infections (HAIs) to hospitals ranges from US $28 billion to $45 billion annually. In nursing homes, an estimated 1.6 to 3.8 million HAI occur annually with a cost ranging from $38–$137 million for antimicrobial therapy and $637 million to $2 billion for hospitalizations due to infections each year. CMS has recently announced that it will penalize 800 hospitals for their hospital-acquired condition (HAC) rates by withholding 1% of their total Medicare payments for patients discharged in 2019. Nursing homes now face fines up to $5,000 when cited for lower level infection control deficiencies that were identified on a previous survey and up to $20,000 if cited for infection control findings twice or more in the last two years. Nursing homes can also damage their 5-star rating if they have to many infections which can affect their reputation and referrals from hospitals for new patients.Innovation Details
Intellectual Property Summary
Currently we do not hold any IP around our algorithms or code and treat them as trade secrets but we continually look for IP opportunities.
Clinical Information
The company is currently validating the EYE software in three pilots sites with the goal of enrolling an additional five nursing home facilities for a three month evaluation. The Company would welcome any help in identifying potential sites for this validation. Please feel free to contact me if you can help.
Regulatory Status
Currently infection surveillance software and clinical decision support software are not regulated by the FDA. The FDA published a guidance document in 2017 on the regulation of clinical decision support software and revised it in September 2019. SmartSteward is exempt from the medical device definition contained in this draft guidance.
How we will use the funds raised
Use of funds include:
- Continued development of our SmartSteward ID, SYNC and AI modules
- Development of a virtual customer training program
- Initiation of sales and marketing activities
Thank You
Lets work together to protect the most vulnerable members of our extended family.
Updates
No updates found .
Supporters
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.
Index Score
2
Score
0
Score
1
Likes0
Partners0
Pilots1
Follows-
This campaign has ended but you can still get involved.See options below.
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.




