Floelle Inc.: Treating female stress urinary incontinence
Making 500 million women happy by treating their incontinence without surgery, giving them their lives back.
ACTON, MA United States Women(s) Health Female FoundersAbout our project
The problem we solve: Stress urinary incontinence afflicts 12% of women 18 and over, 500 million women worldwide, and is a major financial drain on women, their insurance companies and governments. 4,000 year old pads leak, and cause skin irritations and discomfort. Today’s women are too busy to do pelvic floor muscle exercises properly, or to sit through daily 30 minute electrical stimulation sessions. The fear of their pad’s urine odor being detected keeps incontinent women from social contacts. The need to always know where the nearest bathroom is keeps them from vacation travel. Elderly incontinent women, no longer able to change their adult briefs, often move in with their daughters for help, and are then sent to nursing homes, alone. We invented a small soft disposable medical device that stays in their urethras to block the unwanted urine leaks. Grandma can return to the bosom of her family. Incontinent women can again enjoy life without worry.
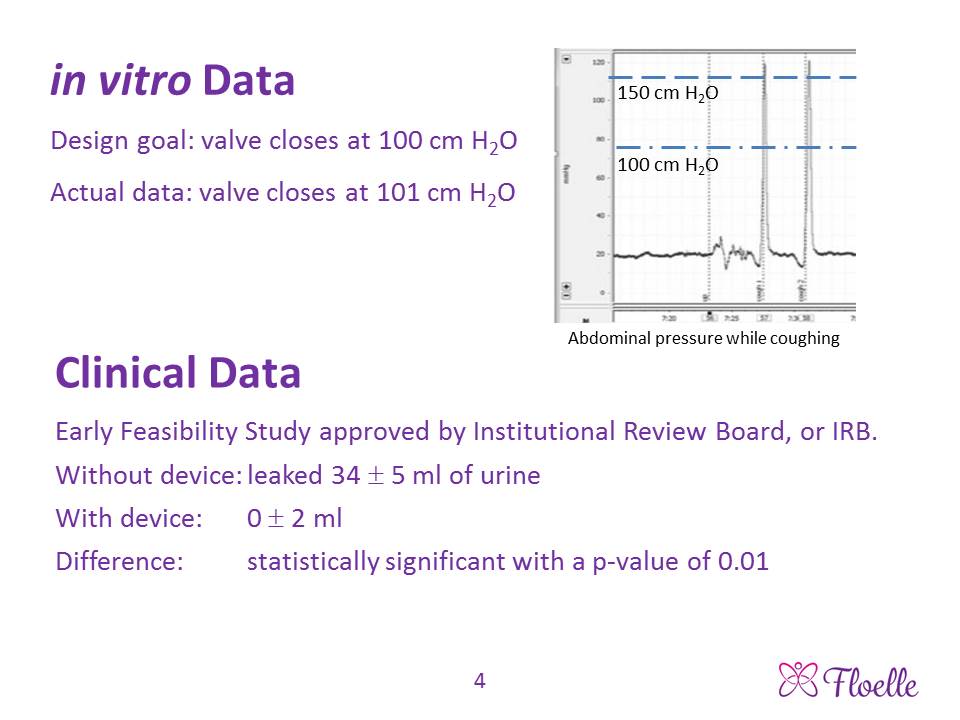
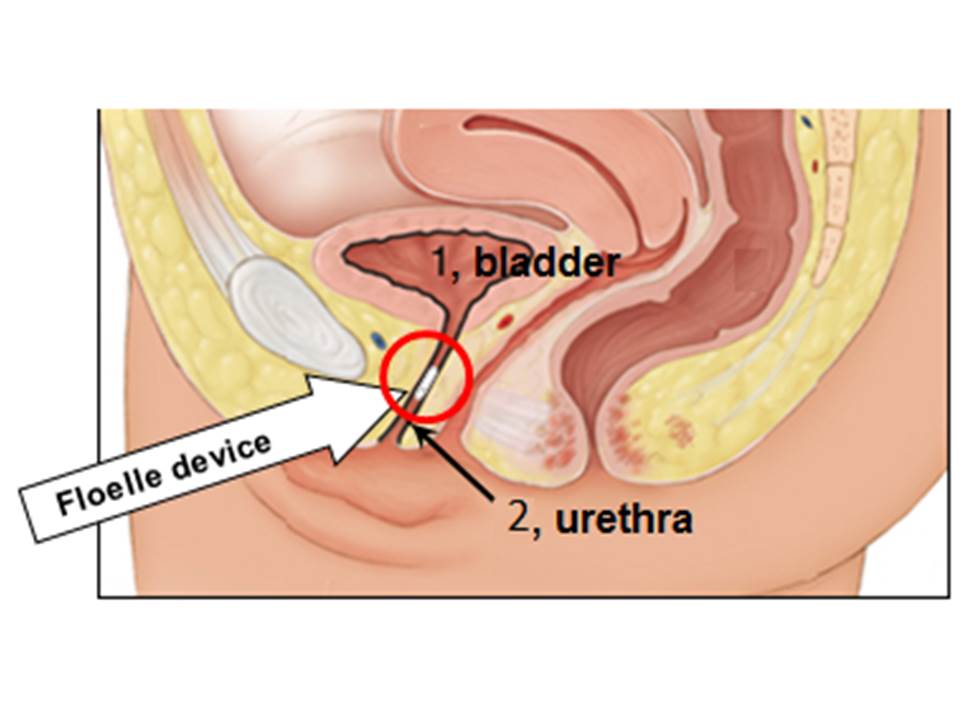
About our solution: Placing a normally open valve in an incontinent woman’s mid-urethra allows normal urination, which we have verified in our Early Feasibility clinical study. The valve was designed to quickly close when the pressure in her abdomen rose past 100 cm of water on its way to the 150 cm of water that occurs during a cough, laugh or sneeze. In vitro tests of five devices showed that the valve shut at an average pressure of 101 cm of water. The Floelle device is an analog computer that constantly senses the pressure against it by the urethra, so uses information technology far more advanced than current software to shut the valve and block the urine leak. Clinical studies have shown that our device reduces the volume of urine leaked in a provocative “pad” test from 34 mL of urine without the device to zero with the device. An antimicrobial mixed into the device’s material prevents bacterial colonization, makes the device disposable and creates valuable revenue for the patient’s physician.
Progress to date:
Progress as of October 19, 2020 The Floelle device has been designed, built, tested in vitro and in vivo and shown to work. Because it is so novel and innovative, it has no predicate, so we are following the FDA de novo 510(k) pathway to regulatory clearance, which will take about two years from significant funding for the pivotal clinical trial and FDA work. We therefore have no sales. The patients in our ongoing Early Feasibility Study all want to take the device home with them, which protocol does not allow. 96% of incontinent women interviewed want our device. We are raising $1.1 million now to get to regulatory approval in the US and Europe.
About Our Team

Creator: Jerrold Shapiro
Location: Massachusetts
Education: PhD from the University of Michigan
Bio: Dr. Shapiro was the first person at Boston University with an earned doctorate in Bioengineering, cofounder of their Department of Biomedical Engineering, Principal Investigator on many NIH grants, Director of two laboratories in glaucoma and ocular physiology and held the positions of Research Scientist, and Associate Professor of Surgery, Medicine, Ophthalmology and Biomedical Engineering. After transitioning to industry in 1990, he was Ophthalmology Program Manager, Director of Engineering, Director of Operations, Chief Operating Officer, CEO, and President of medical device companies. Through MIT, he mentors medical device companies in Portugal. He is co-inventor of an effective treatment for a debilitating medical condition affecting about 500 million women worldwide and leader of the startup medical device company bringing it to market. When he was 8, his IQ was measured and he was enrolled in the Intellectually Gifted Child Program. At age 9, he won 3rd prize in the New York City Science Fair; when he was 11 he won 1st prize in that same venue. He took grades 7 through 9 in two years, and was accepted into the #1 science and math high school in the US, where he was part of the team that designed and built a particle accelerator. He fulfilled his ROTC military obligation by working in the lab that designed the ARPAnet, now called the Internet. He won the Army Commendation Award for inventing holographic character recognition. He applied that same laser technology to detecting breast cancer in mammograms at the speed of light, and then used similar phase gratings to develop precise three dimensional maps of the human eye’s optic nerve for detecting the axonal death from glaucoma. He later invented a way to make a thermal light source behave like a laser to greatly reduce the size and cost of the equipment to make it practical for adding to the fundus camera all eye professionals have in their offices.
Hospital Affiliation: 1974-1990 Assoc. Prof. of Biomedical Engineering, Surgery, Medicine and Ophthalmology at Boston Univ
Title: President
Advanced Degree(s): MSEE, MS Bioengineering, PhD Bioengineering
About Team Members
Cheri Grantham
Vice President, Medical Assistant
Biography: Ms. Grantham has over 20 years of experience in Urodynamics, Pelvic Floor Therapy and Interstitial Cystitis testing. She discovered her calling at the age of five to help people with urinary incontinence when she would see older people in nursing homes wearing diapers. She remembers tugging on her grandmother’s skirt saying, “One day, I am going to help these people.” Determined to find a way to help people with urinary incontinence, she became a bladder specialist who specialized in pelvic floor therapy, treating and running diagnostic testing to improve patients’ quality of life. She has successfully treated hundreds of women with urinary incontinence. Cheri serves as Vice President of Floelle Inc., a startup in Boston developing a novel, minimally invasive medical device for treating female urinary incontinence. . She conceived the initial concept for the Floelle device, and is co-inventor with Dr. Shapiro on all Floelle patents.
Title: Vice President
Advanced Degree(s): Medical Assistant
LinkedIn:
https://www.linkedin.com/in/cheri-grantham-m-a-9a1b8628/
Judy Isaacson
Chief Marketing Officer, B.A., M.S.
Biography: Judy brings over 35 years of experience in medical marketing, communications, investor and public relations. Her specialty is working with small- to medium-sized businesses (SMBs), and startups, increasing overall brand awareness and market share.
Judy is currently Executive Consultant at Vital Now!, an independent consultancy where she helps medical organizations in the areas of corporate branding, medical education, and medical communications.
Prior to Vital Now!, Judy was Vice President of Marketing at Medical Support Systems, a Database Publishing Group company, where she worked extensively with numerous professional medical associations and their pharmaceutical sponsors in producing continuing medical education for a various medical disciplines, namely, hematology, gastroenterology, cardiology, ophthalmology, to name a few.
Prior to Medical Support Systems, Judy was President of Effective Corporate Communications Inc., a boutique full-service marketing communications/public relations agency where she independently created a business model for developing corporate identities which led to follow-on programs in collateral development, advertising, lead generation, and web development, and public relations programs.
Prior to Effective Corporate Communications Inc., Judy was Director of Marketing and Corporate Communications at AST Products, Inc. in Billerica, MA. Prior to AST Products, Inc., Judy was Manager of Marketing and Investor Relations at Spire Corporation.
Judy is a former adjunct lecturer at several Boston-area colleges. Judy received her Bachelor of Arts degree from Emmanuel College and her Master of Science degree from Simmons College in Communications Management. Her graduate thesis addressed “The Importance of a Corporate Image.”
Title: Chief Marketing Officer
Advanced Degree(s): B.A., M.S.
LinkedIn:
https://www.linkedin.com/in/judyisaacson/
Peter Fuchs
Chief Financial Officer, B.S. Economics, M.B.A. Finance and Marketing
Biography: Peter is a Financial Executive with international, business development and general management experience in fast growing entrepreneurial environments. He has demonstrated expertise in budgeting, forecasting, acquisitions and subsequent integration. Peter is a strong business partner skilled at attracting and retaining key contributors.
Specialties: Mergers and acquisitions, Budgeting and forecasting, Restructurings and International business
For nearly three years he has been a partner at Edge Medica LLC, a group of Global Operating consultants /investors serving a wide range of medical device companies. He previously worked as Director of Finance/International at Smith & Nephew, where he was the financial lead for their Endoscopy business unit with sales and marketing operations in Europe, Asia and Latin America. ( 21 operating units ) He lived in Hong Kong for two years while VP of Finance and Administration for the Swatch Group, and is very familiar with how business is done in Asia.
Title: Chief Financial Officer
Advanced Degree(s): B.S. Economics, M.B.A. Finance and Marketing
LinkedIn:
https://www.linkedin.com/in/peter-fuchs-06082a2/
About Our Company

Floelle Inc.
Location: 100 Powdermill Road
Suite 321
ACTON, MA 01720
US
Founded: 2010
Website: https://Floelle.com
Product Stage: Prototype/MVP
YTD Sales: Working on it
Employees: 1-2
How We Help Patients
Dr. Shapiro learned what life is like for incontinent patients during inservice training of their doctors. Busy younger women have to take extra time to replace their incontinence pads every time they urinate. Women starting relationships worry about the surrounding urine odor. Older women worry how wearing adult briefs distorts their clothing to reveal their secret. Women no longer able to change their absorbent garments ten times a day have to move in with their children, and are then sent to a nursing home, all alone. Female swimmers were embarrassed in front of everyone in the pool as urine ran down their leg while they talked to the lifeguard. Women's wet pads irritated their thighs as they walked, causing infections and ulcers. By eliminating the need for absorbent products and restoring continence, Floelle will give all these women their lives, and their dignity, back, free of worry, living with their families, and free to travel without knowing where every ladies room is.
How We Help Physicians
Faced with declining reimbursements and increasing malpractice premiums, health care providers are looking for new revenue sources from using devices that improve their patients’ lives. The existing CPT codes cover the removal of the old Floelle device and insertion of its replacement; these are reimbursed $500 by Medicare, usually more by private insurance, and provide continuing annual revenue of $1,500 per SUI patient, and $180,000 of new annual revenue per doctor in a typical practice.
Innovation Details
Intellectual Property Summary
U.S. Patent No’s.10,610,344 and 9,510,924 have issued
Japanese Patent No. 6026503 has issued
EU patent will issue in December 2020
Clinical Information
An IRB approved Early Feasibility Study, EFS, of the Floelle device is underway. The study sponsor declared it a NonSignificant Risk, NSR, study as it involves inserting the Floelle device into the mid-urethra of urinary incontinent female subjects for less than two hours while provocative tests measure urine leakage without, then with, the device in place. The device is withdrawn after the study is complete and retained by the sponsor. The study in Houston, Texas was delayed for five months by COVID-19 lockdowns, and restarted on August 31st. The IRB is Asentral IRB in Newburyport, MA and it approved Floelle Protocol FS01 as IRB #2018-522B Balat on November 5, 2019. Asentral IRB was later purchased by BRANY.
The clinical data indicate that the Floelle device works as designed.
As the Floelle device is unique and highly innovative, it has no predicate. We are following the FDA’s de novo 510(k) regulatory pathway under the guidance of a regulatory affairs expert, who trains the FDA.
We can only accept financial help from accredited investors who can invest via our convertible promissory note, with a $25,000 minimum.
Regulatory Status
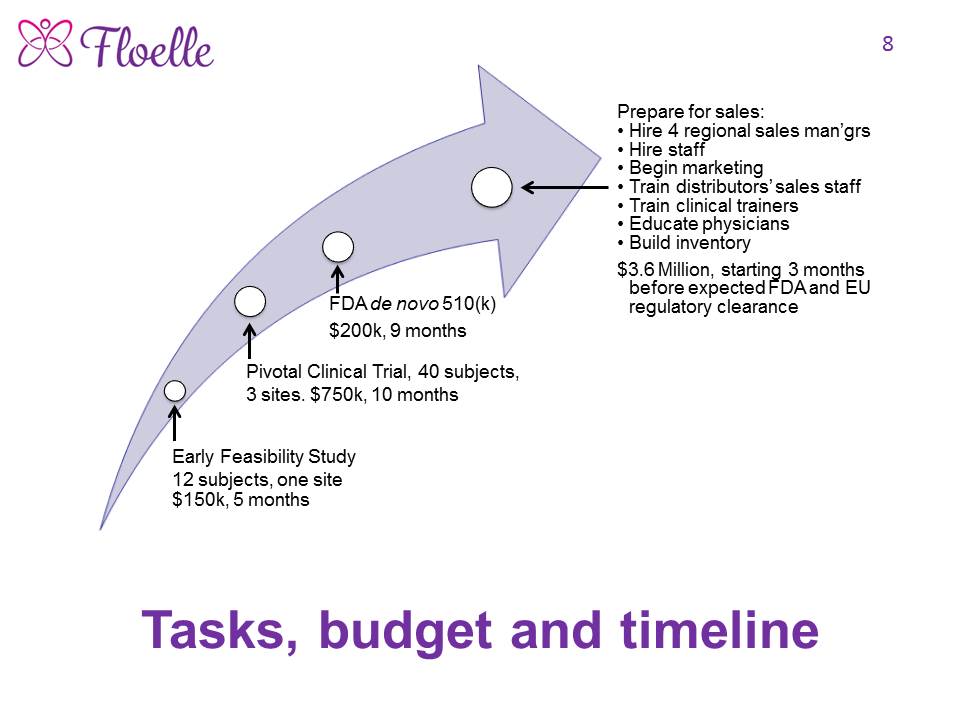
As the Floelle device is unique and highly innovative, it has no predicate. We are following the FDA’s de novo 510(k) regulatory pathway. Since Asentral IRB approved our Early Feasibility Study, EFS, as one with NonSignificant Risk, we did not need an IDE to do the study. We are raising $1.1 million to complete the EFS, run a multicenter international pivotal clinical trial, and secure regulatory clearances by the FDA and the European authorities.
How we will use the funds raised
We need $150,000 to complete the Early Feasibility Study and any necessary engineering changes that result; $750,000 to design, run and analyze the data from a multisite international pivotal clinical trial; and $200,000 to prepare, submit and get regulatory clearance by the FDA via their de novo 519(k) pathway, and the Class 2b pathway in Europe.
Thank You
Help us make 500 million women worldwide happy by treating their urinary incontinence with a tiny medical device, letting them enjoy life without worry. Bring grandma's, exiled to nursing homes because of the need to change their wet diapers ten times a day, home to enjoy being with their children and grandchildren. Save the environment by keeping nearly two Trillion wet diapers out of landfills. And decrease your taxes by saving our governments trillions of dollars each year now spent keeping incontinent women in nursing homes.
Updates
No updates found .
Supporters
-
 Instant Feedback
Instant Feedback
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.
2Medstartr
Index Score2
Interest
Score0
Adoption
Score2
Likes0
Partners0
Pilots1
Investors-
This campaign has ended but you can still get involved.See options below.
$25,000 pledged of $1,100,000 goal$25,000 Investor, Pilot & Parnter
interest to date.
Instant Feedback
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.
-