SPMF Therapy: Non invasive technology to treat Osteoarthritis and Cancer

SPMF (Sequentially Programmed Magnetic Field) therapy uses non-invasive, targeted magnetic fields to treat spinal disorders by promoting cellular repair and reducing inflammation non-invasively.
Bangalore, KA India Cancer Care Orthopedics Medical DeviceAbout our project
The problem we solve: SPMF (Sequentially Programmed Magnetic Field) therapy addresses the growing burden of chronic musculoskeletal disorders and certain cancers, notably brain tumors. Globally, arthritis affects over 350 million people, with osteoarthritis being the most common form, especially in aging populations. Spinal arthritis and degenerative disc disease are leading causes of disability and chronic pain. Additionally, cancers involving the spine, such as metastatic spinal tumors, are on the rise due to increased cancer survivorship and aging. Conventional treatments like surgery, radiation, and medication often have limitations, side effects, or are not viable for all patients. SPMF therapy offers a non-invasive, safe, and targeted alternative by using low-intensity magnetic fields to stimulate cellular repair, reduce inflammation, and slow disease progression. It fills a critical need for patients seeking effective, non-surgical solutions for managing spinal arthritis and related
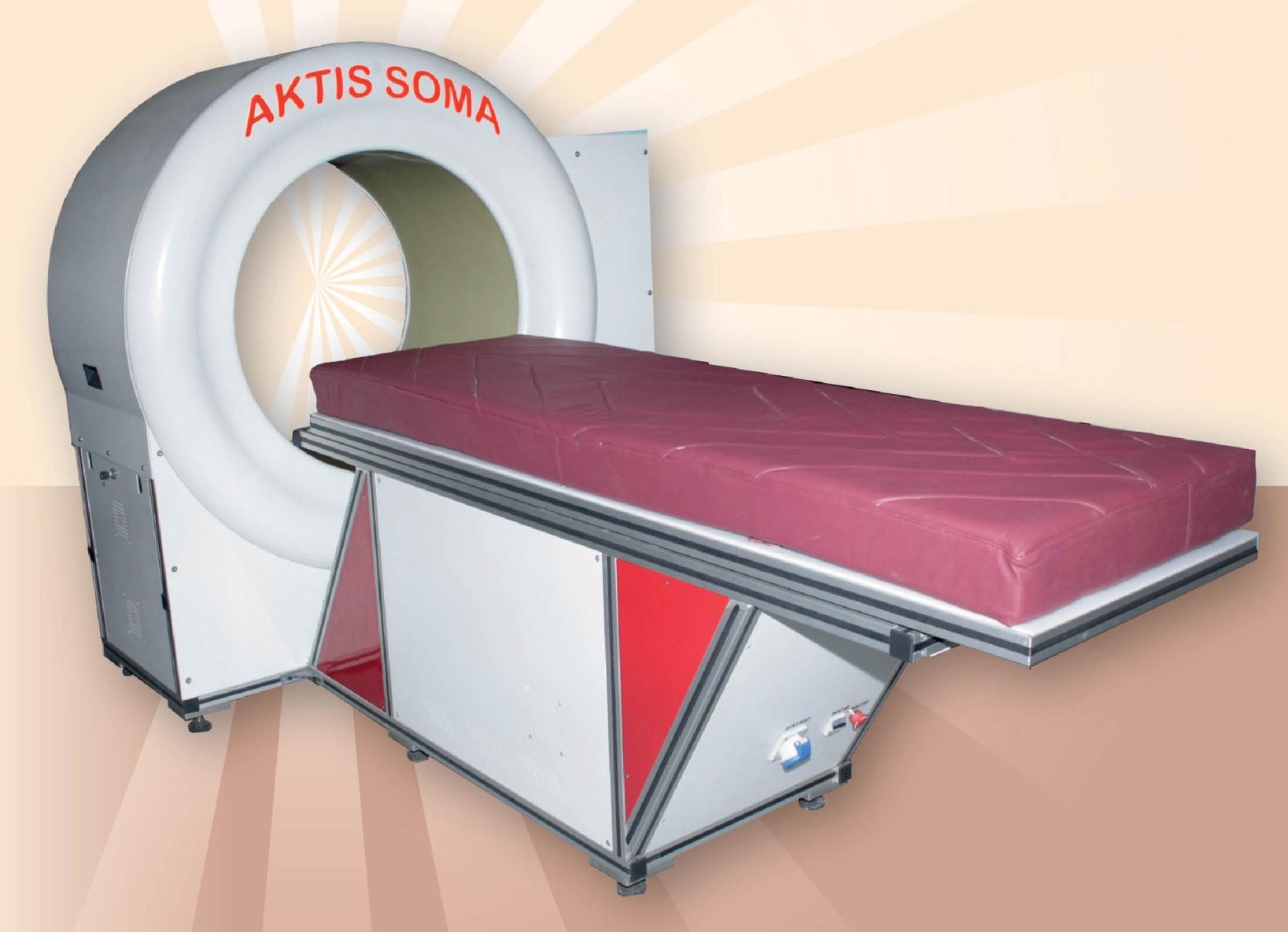
About our solution: The therapy uses the technology of Sequentially Programmed Magnetic Fields (SPMF) and has been pioneered and patented by Late WG CDR (Dr) V G Vasishta who was a professor and head at the institute of Aerospace Medicine, Indian Air Force. Based on sound scientific principles which tackle the root cause of cartilage degeneration through the use of non-ionizing radiation, SPMF-Therapy treatment not only stops the progress of the disease but also reverses the process of disease by cartilage regeneration. Similarly, SPMF exposure to cancer cells causes apoptosis by sensitizing the cancerous cells and normalizes the cell membrane potential. It halts the process of cell proliferation leading to apoptosis. SPMF-Therapy is delivered by the device AKTIS SOMA® invented by Dr. Vasishta who has published patents for the same in India, USA and also has a worldwide PCT.
Progress to date:
Sequentially Programmed Magnetic Field (SPMF) therapy is a non-invasive, painless, and drug-free treatment patented by SBF Healthcare, primarily for osteoarthritis and certain cancers. It utilizes computer-controlled, low-frequency magnetic fields to stimulate cellular regeneration in affected tissues, such as cartilage in joints, and induce apoptosis (programmed cell death) in cancer cells while sparing healthy ones.
This therapy aims to address the root cause of conditions like osteoarthritis by promoting cartilage regeneration and halting disease progression. For cancer, it normalizes cell membrane potential, inhibiting proliferation.
SBF Healthcare reports having treated over 10,000 patients with a reported success rate of about 85%, significantly reducing pain and improving mobility for osteoarthritis and improving survival/quality of life for cancer patients. Clinical trials have been conducted, with positive results for conditions like recurrent Glioblastoma's and osteoarthritis, demonstrating tumor size reduction and functional improvements. Further trials are being initiated for other degenerative conditions like diabetes and Alzheimer's.
About Our Team

Creator: Leena Vasishta
Location: Katanga
Bio: Leena Vasishta stands at the forefront of healthcare innovation, making significant strides in the sector. Managing Director, SBF Healthcare: As the MD of SBF Healthcare, a leading health tech organization, Leena has spearheaded groundbreaking initiatives in the fields of orthopedics and oncology. Under her visionary leadership, SBF Healthcare has developed and implemented innovative technologies and treatments, significantly improving patient outcomes and transforming traditional healthcare practices. Notably, SBF Healthcare is one of the first companies in the world to develop: • Non-Surgical Treatment for Osteoarthritis: A non-surgical and non-invasive treatment for osteoarthritis by regenerating cartilage cells. This pioneering approach has opened new avenues for treating a condition that affects millions globally, offering hope and improved quality of life to many. • Non-Surgical Cancer Treatment: A non-surgical treatment for cancer using targeted radio frequencies programmed to induce apoptosis (programmed cell death) of cancer cells. This innovative treatment represents a significant breakthrough in oncology, providing a less invasive option for cancer patients. Vision and Mission: Leena Vasishta is driven by a mission to revolutionize healthcare through innovation. She envisions a future where advanced health tech solutions are accessible to all, and where patient care is continually enhanced through technological advancements. Leena’s multifaceted approach to leadership, her dedication to societal betterment, and her relentless pursuit of excellence make her a distinguished figure in the healthcare sector. Leena Vasishta continues to inspire and lead by example, paving the way for a brighter, healthier world.
Title: Director
About Our Company

SBF Healthcare and Research Centre
Location: Sri kote asirwad towers
1st floor
Bangalore, KA 560037
IN
Founded: 2006
Website: https://sbfhealthcare.com/
Facebook: https://www.facebook.com/share/16UTtZE37m/?mibextid=wwXIfr
Other link: https://www.instagram.com/sbf_healthcare?igsh=d3lpdG5idzEycWZ2
Product Stage: In the Market
Employees: 3-5
How We Help Patients
SPMF therapy profoundly improves patients' lives by offering a non-invasive, drug-free, and painless treatment with no known side effects, directly contrasting with the inherent risks of surgery.
For arthritis and degenerative disc diseases, it provides genuine relief by regenerating damaged cartilage, reversing disease progression. This restores mobility and reduces chronic pain, enabling an active lifestyle without needing invasive surgeries. For instance, total knee replacement carries 30-day mortality rates around 0.14-0.35%, and total hip replacement around 0.3-0.5% at 30 days, with 1-year mortality rates of 1-2% for both. Spinal fusion surgery can also have a mortality rate, reported as around 0.13% overall for lumbar spine surgeries, increasing for complex procedures.
In cancer treatment, SPMF therapy offers a beacon of hope by selectively inducing apoptosis (programmed cell death) in cancer cells, leaving healthy cells unharmed. This non-surgical approach can halt disease progression, improve overall quality of life, extend survival periods, and reduce the harsh side effects associated with conventional treatments. In contrast, cancer surgeries can have mortality rates varying significantly based on cancer type, stage, and healthcare setting, with some major gastrointestinal cancer surgeries reporting 30-day mortality rates of 2-10% or even higher in lower-income countries.
Ultimately, SPMF empowers patients to live fuller, more comfortable lives, free from the debilitating symptoms, arduous recoveries, and the inherent mortality risks often linked to surgical interventions.
How We Help Physicians
SPMF therapy offers significant benefits to healthcare providers, especially physicians, by expanding their therapeutic arsenal with a non-pharmacological, non-invasive, and scientifically supported modality, addressing crucial clinical challenges.
For orthopedic and rheumatology specialists, SPMF is a viable alternative to conventional pharmacotherapy and invasive surgical interventions for joint degeneration. It leverages sequentially programmed magnetic fields to modulate cellular bioenergetics and signal transduction pathways, promoting chondrocyte proliferation and extracellular matrix synthesis, thereby facilitating cartilage regeneration in osteoarthritis (OA) and degenerative disc disease (DDD) [SBF Healthcare patents and publications by Vasishta VG]. This solves the pervasive problem of progressive joint destruction that current treatments often only ameliorate symptomatically or through invasive means associated with significant morbidities (e.g., surgical site infection, thromboembolic events). Physicians can now offer a regenerative approach that profoundly improves joint function, reduces chronic pain, and restores mobility without the inherent risks of surgery (e.g., 30-day mortality rates of ~0.14-0.35% for total knee replacement, and ~0.3-0.5% for total hip replacement; ~0.13% overall for lumbar spine surgeries). This empowers physicians to offer a safer, less disruptive path to improved quality of life.
For oncologists, SPMF therapy provides a novel adjunctive or standalone treatment. Its proposed mechanism involves inducing selective apoptosis in neoplastic cells through normalization of aberrant cellular electromagnetic fields, disruption of mitotic spindle dynamics, and activation of tumor suppressor pathways like p53 [SBF Healthcare publications; e.g., "Sequentially Programmed Magnetic Field Therapy in the Management of Recurrent Anaplastic Astrocytoma: A Case Report and Literature Review," Case Reports in Oncology, 2010]. This offers a critical solution for managing advanced or refractory cancers, potentially reducing tumor burden, enhancing radiosensitivity/chemosensitivity, and mitigating treatment-related toxicities. Physicians can now offer a therapy that specifically targets cancer cells while largely sparing healthy tissue, addressing the fundamental challenge of systemic toxicity in conventional chemotherapy and radiation, which can have mortality rates varying significantly (e.g., 2-10% or higher for some major cancer surgeries).
Furthermore, SPMF helps physicians address unmet patient needs for those unsuitable for or refusing surgery/harsh treatments, expanding their ability to provide effective care. Its non-invasive nature often leads to enhanced patient compliance and satisfaction, improving overall treatment adherence. Finally, incorporating SPMF therapy allows providers to offer a specialized, cutting-edge service, differentiating their practice and attracting new patients seeking advanced, low-risk solutions, underpinned by extensive clinical experience (over 10,000 OA cases treated by SBF Healthcare).
In essence, SPMF therapy empowers physicians with a safe, efficacious, and patient-centric tool to address complex pathologies, leading to improved patient outcomes and reduced healthcare burden, while concurrently bolstering their practice's capabilities and reputation.
How We Help Hospitals
SPMF therapy offers multifaceted advantages to hospitals, significantly benefiting patient care, operational efficiency, and financial health.
-
Enhanced Clinical Service Portfolio & Patient Attraction: Hospitals can integrate a cutting-edge, patented, non-invasive treatment for high-volume conditions like osteoarthritis and cancer. This expands their service offerings beyond conventional surgery and pharmacotherapy, attracting patients seeking advanced, less debilitating alternatives. The proven ability of SPMF to regenerate cartilage (as seen in over 10,000 OA cases by SBF Healthcare) and induce selective cancer cell apoptosis positions the hospital as a leader in innovative, restorative care. This directly addresses the evolving patient demand for safer, effective, and less invasive options.
-
Reduced Surgical Burden & Associated Risks: By offering SPMF, hospitals can significantly reduce the volume of elective orthopedic surgeries (e.g., knee and hip replacements) and potentially some complex cancer resections. This translates directly to fewer surgical complications (e.g., infections, DVT, pulmonary embolisms, readmissions), leading to lower associated costs for managing these complications. Furthermore, it decreases strain on critical hospital resources like operating rooms, ICU beds, and specialized surgical and post-operative nursing staff, optimizing overall departmental efficiency.
-
Improved Patient Flow and Outpatient Capacity: SPMF therapy is primarily an outpatient procedure, typically requiring daily sessions over a few weeks. This maximizes the efficient utilization of hospital infrastructure as it doesn't necessitate inpatient beds, prolonged recovery unit stays, or extensive post-operative care. This allows for a higher patient throughput within existing facilities and optimizes the allocation of valuable inpatient resources for more acute or complex cases.
-
Positive Reputation and Enhanced Patient Satisfaction: Offering a treatment with no known side effects, significant pain reduction, and genuine regenerative outcomes (in arthritis) or extended survival/improved quality of life (in cancer) leads to measurably higher patient satisfaction scores. This dramatically enhances the hospital's reputation within the community and among referring physicians, fostering positive word-of-mouth referrals and increasing long-term patient loyalty – a crucial asset in today's competitive healthcare landscape.
-
New Revenue Streams and Favorable Cost-Benefit: Implementing SPMF can generate new revenue streamsthrough direct treatment fees. While an initial investment in the SPMF device (like AKTIS SOMA®) is required, the reduced long-term costs associated with preventing complex surgeries, shorter overall treatment durations (compared to prolonged conventional therapies), and fewer complication-related readmissions present a highly attractive cost-benefit ratio for the hospital over time. This makes SPMF a financially sound investment for sustainable growth.
In essence, SPMF therapy allows hospitals to deliver superior patient outcomes with reduced risks and resource intensity, while simultaneously enhancing their market position, reputation, and financial viability, adapting to the future of healthcare.
How We Help Partners
SPMF therapy presents an exceptional opportunity for business partners, capitalizing on a significant market shift towards less invasive, more patient-friendly treatments. As a new, patented, and highly effective treatment offering, it addresses a burgeoning demand that traditional medicine often struggles to meet.
A growing number of individuals are actively opting out of surgery for conditions like arthritis and cancer, driven by concerns about invasiveness, prolonged recovery times, potential complications (including mortality risks associated with major surgeries), and the overall impact on quality of life. For instance, in India, while knee replacements are common, many arthritis patients, especially younger ones, are increasingly exploring options to delay or avoid surgery. Similarly, for cancer, patients often face immense financial and physical burdens from conventional treatments, leading some to seek alternatives.
SPMF directly caters to this demographic. Business partners introducing this therapy can offer:
- Market Differentiation: Position themselves as pioneers in offering a state-of-the-art, non-invasive alternativethat truly regenerates tissue (in arthritis) and selectively targets cancer cells. This unique selling proposition attracts a significant patient cohort disillusioned with existing options.
- Expanded Patient Reach: Tap into the large population actively avoiding surgery due to fear, health comorbidities, financial strain, or a desire for holistic healing. This opens up a new, underserved patient base.
- Faster Patient Throughput: As an outpatient therapy, SPMF allows for efficient scheduling and higher patient volumes compared to inpatient surgical procedures, optimizing operational efficiency and revenue per square foot.
- Strong Referral Network: The therapy's compelling clinical outcomes and non-invasive nature encourage referrals from physicians and other healthcare professionals seeking better solutions for their patients.
- High Patient Satisfaction & Retention: Patients appreciate the lack of pain, quick recovery, and significant improvements in their conditions, leading to positive testimonials and repeat business, fostering a loyal patient base.
By partnering with SPMF, businesses can enter a lucrative segment of the healthcare market, providing a humane, effective, and in-demand treatment that aligns perfectly with the evolving preferences of modern patients seeking alternatives to surgical intervention.
Innovation Details
Intellectual Property Summary
Patented in the US, Europe and India
Patent Link
https://patents.google.com/patent/US9278231B2/en?oq=US+-+9278231
Clinical Information
SPMF Therapy: Our Path to US FDA Approval
The US urgently needs novel therapies for devastating conditions like Glioblastoma Multiforme (GBM) and severe Osteoarthritis. SPMF (Sequentially Programmed Magnetic Field) Therapy, a non-invasive, drug-free solution from SBF Healthcare, directly addresses this.
Our AKTIS SOMA® device delivers precisely programmed magnetic fields. For cancer, it aims to normalize cell membrane potential, inducing apoptosis. For osteoarthritis, it seeks to regenerate cartilage and reduce inflammation. Our experience in India shows a strong safety profile.
Our US FDA strategy is clear:
Rigorous Clinical Trials & Regulatory Navigation
- Foundation: Leveraging early MoUs with UCLA and the University of Pennsylvania, we've laid a strong scientific groundwork.
- IDE & Classification: We're pursuing an Investigational Device Exemption (IDE) after determining appropriate device classification (likely Class II/III).
- Trial Design: We're designing multi-center, randomized controlled trials (RCTs) in the US, adhering to GCP.
- GBM Endpoints: Focusing on overall survival (OS), progression-free survival (PFS), and quality of life (QoL).
- Osteoarthritis Endpoints: Measuring pain reduction, functional improvement, and cartilage regeneration via imaging.
- FDA Engagement: Proactive pre-submission meetings will guide our strategy. We aim for expedited programslike Fast Track or Breakthrough Therapy designation, given the unmet medical needs.
- Submission: Post-trial, a comprehensive 510(k) or PMA submission will be prepared.
Commitment to US Healthcare
We're committed to post-market surveillance and maintaining a robust Quality Management System (QMS). Our investment in US clinical trials reflects our dedication to bringing this transformative therapy to American patients. We seek strategic partners and investment to accelerate our FDA journey and market launch.
Join us in transforming lives.
Regulatory Status
The FDA approval process for SPMF therapy involves key steps for medical devices. First, device classification (Class I, II, or III) determines the regulatory pathway. SPMF, likely a Class II or III device due to its intended use for serious conditions, would require an Investigational Device Exemption (IDE) for clinical trials.
These US-based trials must adhere to Good Clinical Practice (GCP), with rigorous design, endpoints (e.g., survival, pain reduction, cartilage regeneration), and robust data collection. Proactive pre-submission meetings with the FDA are crucial. Depending on preliminary data, SPMF might qualify for expedited programs (Fast Track, Breakthrough Therapy).
Finally, a comprehensive Premarket Submission (510(k) or PMA), including all clinical and manufacturing data, is required for market clearance. Post-market surveillance ensures ongoing safety.
How we will use the funds raised
Funds will primarily drive FDA approval and clinical trials for SPMF therapy in the US. This includes:
- Regulatory Expertise: Hiring consultants to navigate device classification (likely Class II/III) and prepare the Investigational Device Exemption (IDE) application.
- Clinical Trial Execution: The largest expense. This covers multi-center RCT design (GCP-compliant), site selection, patient recruitment, data management, statistical analysis, and safety monitoring by a DSMB. Funds will also cover manufacturing/supplying AKTIS SOMA® devices for trials and patient-related costs (e.g., imaging).
- FDA Fees: Paying substantial user fees for pre-submission meetings, and the final Premarket Submission (510(k) or PMA). (FY2024 fees: 510(k) ~$21,760; PMA ~$483,560).
- Quality Systems: Establishing an FDA-compliant Quality Management System (QMS).
These investments are crucial to generate the evidence needed for FDA clearance and bring SPMF to US patients.
Thank You
We stand at a pivotal moment, on the cusp of bringing SPMF Therapy to patients in the US who desperately need new hope. This isn't just about a medical device; it's about giving a fighting chance to those battling aggressive brain tumors and providing relief from debilitating osteoarthritis.
The path to FDA approval and successful clinical trials is rigorous and resource-intensive. We've seen the incredible potential of SPMF therapy firsthand, but turning that potential into accessible treatment requires substantial funding. Your support is crucial to cover complex trial design, patient recruitment, advanced data analysis, and the significant regulatory fees that stand between us and widespread availability.
Imagine the lives we can transform – alleviating pain, extending precious time, and offering a renewed sense of hope. Your contribution isn't just an investment; it's a profound act of compassion, directly enabling us to bring this life-changing therapy to those who need it most. Help us heal, help us make a difference.
Supporters
There are not supporters yet.
Updates
No updates found .
0
Score
0
Score
0
Likes0
Partners0
Pilots0
Follows-
11 Days left
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.
Important Disclosure: MedStartr.com is a website owned and operated by MedStartr, Inc., which is not a broker-dealer, funding portal or investment advisor; and neither the website nor MedStartr, Inc. participate in the offer or sale of securities. All securities related activity is conducted through Young America Capital, LLC, a registered broker-dealer and member of FINRA/SIPC. No communication, through this website, email or in any other medium, should be construed as a recommendation for any securities offering.
