The Glucose Hawk: Glucose and Cancer Monitoring Project
The goal of our project is to monitor Glucose and Cancer non-invasively with the short range goal of monitoring electrolytes such as Potassium, Magnesium and Sodium levels.
San Diego, CA United States Wearables Diagnostics Diabetes MedStartr Ventures challengeAbout our project

The problem we solve: Our technology solves the problem of detecting and measuring the most complex human diseases non-invasively and sending the information remotely. The greatest advantage to the use of this technology is that it can be employed to detect and measure various types of blood content including, but not limited to electrolytes, metabolites, and small molecules such as amino Acids, DHEA and cortisol. We will also be able to detect proteins such as interleukins, tumor necrosis factor, and neuropeptides.

About our solution: Our technology is geared to detect specific signatures of glucose emitted from the epidermal layer of skin and is able to measure its exact levels in the blood from there. This can all be done on one device not much bigger than a quarter that is placed directly on the skin. The greatest advantage to the use of this technology is that it can be employed to detect and measure various types of blood content including, but not limited to electrolytes, metabolites, and small molecules such as amino Acids, DHEA and cortisol. We will also be able to detect proteins such as interleukins, tumor necrosis factor, and neuropeptides. We expect this technology to not only enable the creation of devices that will take over the CGM market, but also to change the scope of detection for various medical conditions around the world. In the process, with this technology we may even be able to end the need for blood draws forever. We are currently in a three stage prototyping process
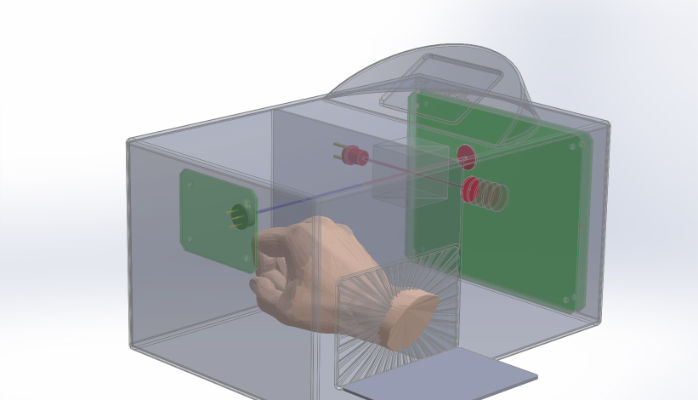
Progress to date:Completed stage 1, process of completing stage 2 our shoebox-like concept chamber, which will be able to perform a majority of our diagnostic processes accurately with the ability to assign numerical values to signature measurements of glucose and more. Our chamber will be a single transportable device suitable for demonstration purposes and licensing. This build contains a chamber with a gasket sealed opening which will allow entry of a human hand for readings in addition to controlled samples. Moreover, the chamber will be in the form of a clear shoebox which will be located at Rite Aids and CVS stores. It will have the ability to replace the blood pressure reading devices at these stores giving the customer ability to read blood pressure, glucose, cholesterol, and electrolytes, such as potassium, magnesium and sodium. Importantly, our shoebox chamber will be able to be sold in hospitals, drug stores and in residential markets. Stage 2 precedes miniaturization to wearable.
About Our Team

Creator: George McKinney
Bio: My name is George McKinney and I have been an innovator and a tinkerer for most of my life. Last year I received the Roy Clay Award for being one of the top 50 African-Americans in technology our diabetic monitoring concept. In addition our technology just received a major endorsement from the University of Arkansas Pine Bluff which will dedicate faculty and staff to our technology once funded. We have a patent on a powerful wireless technology, as well as, patent pending new tech.
Title: President/CEO
About Team Members
Dr. Robert Walker
Executive Vice President, Doctor of Osteopathic Medicine
Biography: Walker graduated from Michigan State University, College of Osteopathic Medicine. He has expertise in the field of research and development and has earned recognition as an experimental researcher from the Department of Psychology, University of California, Berkeley. He has been one of the foremost researchers in the area of cancer at Cal Berkeley and is presently Chief Medical Officer at Donovan heading Diabetic facilities with a billion dollar budget. Dr. Walker is a co-inventor.
Title: Executive Vice President
Advanced Degree(s): Doctor of Osteopathic Medicine
LinkedIn:
https://www.indiegogo.com/projects/wireless-wearable-non-invasive-glucose-monitor-cancer-technology--3/x/8424087#/
David Nichols
Director of Mechanical Technology, Mechanical Engineering
Biography: Nichols has over 30 years expertise in engineering management, project management, concept design, mechanical systems design, test fixtures, validation, 3D solid modeling, industrial design, rapid prototyping, material selection, fabrication, manufacturing, production coordination, and production tooling (injection molding, thermo forming, extrusion, and sheet metal operation). Nichols has extensive knowledge in working with medical devices with SAIC.
Title: Director of Mechanical Technology
Advanced Degree(s): Mechanical Engineering
LinkedIn:
https://www.indiegogo.com/projects/wireless-wearable-non-invasive-glucose-monitor-cancer-technology--3/x/8424087#/
Glenn Battle
Project Lead, Electrical and Mechanical PHd. Candidate at SDSU
Biography: Glenn Battle also has over 25 years of engineering experience in design and technology development in the areas of aerospace, advanced computing, IT security, disaster recovery, cloud computing, mobile communications, voice video, data, wearable technologies, medical technology development, and biological sensors. Additionally, he his currently pursing a PhD in geodetic sciences. Glenn is an expert in drone technology and has several patents working on guidance systems and stealth technology.
Title: Project Lead
Advanced Degree(s): Electrical and Mechanical PHd. Candidate at SDSU
LinkedIn:
https://www.indiegogo.com/projects/wireless-wearable-non-invasive-glucose-monitor-cancer-technology--3/x/8424087#/
About Our Company

Better Life Technologies Group, Inc.
Location: 5625 Imperial Ave.
San Diego, CA 92114
US
Founded: 2011
Website: http://betterlifetech.net
Product Stage: Prototype/MVP
YTD Sales: Working on it
Employees: 5-10
How We Help Patients
It is important to note, that the Continuous Glucose Market(CGM) market is in its early stages and is rapidly growing. This is mainly due to a rise in the number of diabetics and numerous technological advancements. The CGM market will be valued at $2.9 Billion by 2021 and we believe that with our GDS device, we stand to capture 20% of this market by that time. The standard test strips which represent billions of dollars in spending per year will become obsolete as these CGM devices become more available, giving consumers more information in real time than ever before. Please keep in mind that the above is just the beginning of the scope of our technology. It will cover much more, and will have the ability to monitor cancer patients, stroke patients, patients with neurological disorders and much much more. We also plan to include marketing to patients in 5000 targeted hospitals, residents of senior facilities, home owners, Alzheimer’s Association members, cardiac patients, also individuals fighting Obesity, Diabetes, & Stroke, and those with, Epilepsy, and fighting Paraplegic and Quadriplegic disorders, Spinal dangers, Sleep Apnea, Thyroid disease, high blood pressure, Cancer, Parkinson's, Blood clots, and individuals at high risk during travel. To sum it up, we plan to do the following:
•We monitor patients in the hospitals and in their homes by transmitting and receiving vital information from their glucose, cholestoral, and electrolytes readings that would be used in weight loss and physical fitness goals, including imaging records, cast wave files and video files which will create better tracking. Imagine an emergency and calling and sending for an ambulance and having the parametics receiving vital information on the patient before they arrive. How powerful would that be? I would be a game-changer. To sum it up, the major accomplishments that our technology promises are the following:
•Give patients the ability to interact with their doctors and their caretakers real time and make needed course corrections in terms of their health.
• Give patients the ability to non-invasively monitor Glucose, Aortic Blood Pressure, Cholestoral Readings, Electrolyte Readings, and much more .
•Saves patient lives and money by creating a superior delivery system for remote health care utilizing advanced wireless technology including a method of incryption that will protect patient information.
Finally, we need your support and will reward your gifts according the the patient reward section. Please understand that we may need FDA approvals prior to giving out samples. However we think we can give your pretty cool stuff for your support. Help us to help humanity!
How We Help Physicians
Unnessary hospital visits and medical false alarms drive up the cost of health care.
•Creating real-time interaction between patients and providers
•Patient Falls: We have developed software and hardware that will be contained within our technology that will monitor patient falls
•Elopement: Elopement can be a problem with Alzheimers patients where they get confusion and wander off from needed care. Glucose Monitoring Device with its GPS system will allow caregivers to track those who may be putting themselves in danger and continue to monitor their health.
•Staff Assistance: The information on our device will can disseminated throughout a care facility allowing easy access to patients may have physical emergencies.
Reporting: Real time recording and reporting of vitality information on a patient, is so important, we truly believe that it is the bread and butter of efficient patient care, this coupled with giving the patient the technology to interact with providers so that they can make meaningful and necessary course corrections based upon instruction coming from their primary care providers. We have developed technology covered in our patent that will give the primary care provider the ability to monitor and interact with as many patients as need be, and give providers a treasure chest of meaningful applications which will increase job efficiency:.
Applications:
People tracking inside and outdoors
Can be adapted for Autistic Patient tracking indoors and outdoors
Can be adapted for Diabetes Patient needs
Can be adapted for Cardiac Patient needs
Can be adapted for a variety of other special needs Patient tracking and trace
Can be adapted for Sleep Apnea Patients
Can be adapted to Alzheimer's Patients
Can be adapted for children and adults with cognitive disabilities to enhance safety
How We Help Hospitals
It is important to note, that the Continuous Glucose Market(CGM) market is in its early stages and is rapidly growing. This is mainly due to a rise in the number of diabetics and numerous technological advancements. The CGM market will be valued at $2.9 Billion by 2021 and we believe that with our GDS device, we stand to capture 20% of this market by that time. The standard test strips which represent billions of dollars in spending per year will become obsolete as these CGM devices become more available, giving consumers more information in real time than ever before. Please keep in mind that the above is just the beginning of the scope of our technology. It will cover much more, and will have the ability to monitor cancer patients, stroke patients, patients with neurological disorders and much much more. We also plan to include marketing to patients in 5000 targeted hospitals, residents of senior facilities, home owners, Alzheimer’s Association members, cardiac patients, also individuals fighting Obesity, Diabetes, & Stroke, and those with, Epilepsy, and fighting Paraplegic and Quadriplegic disorders, Spinal dangers, Sleep Apnea, Thyroid disease, high blood pressure, Cancer, Parkinson's, Blood clots, and individuals at high risk during travel. To sum it up, we plan to do the following:
•We monitor patients in the hospitals and in their homes by transmitting and receiving vital information from their glucose, cholestoral, and electrolytes readings that would be used in weight loss and physical fitness goals, including imaging records, cast wave files and video files which will create better tracking. Imagine an emergency and calling and sending for an ambulance and having the parametics receiving vital information on the patient before they arrive. How powerful would that be? I would be a game-changer. To sum it up, the major accomplishments that our technology promises are the following:
•Give patients the ability to interact with their doctors and their caretakers real time and make needed course corrections in terms of their health.
• Give patients the ability to non-invasively monitor Glucose, Aortic Blood Pressure, Electrolyte readings, and much more .
•Saves patient lives and money by creating a superior delivery system for remote health care utilizing advanced wireless technology including a method of incryption that will protect patient information.
Finally, we need your support and will reward your gifts according the the patient reward section. Please understand that we may need FDA approvals prior to giving out samples. However we think we can give your pretty cool stuff for your support. Help us to help humanity!
How We Help Partners
In the month of August we received Endorsement from the University of Arkansas which is open to use its faculty and staff to build our device from our present prototype, to the ready for market edition, including setting up manufacturing, and facilitating partnerships globally working hand-in-hand with Fallbrook Engineering of Luecacia California. Fallbrook Engineering was established in 1981 and is one of the premire engineering firms in the country specializing in medical device development and manufacturing. Further, Fallbrook is one of the top developers of medical devices doing work for major hospitals such as Scripps La Jolla---They are co-inventors of the latest stent technologies along with Dr. Paul Tierstien MD Chief of Cardiology at Scripps and they do a lot of work with Qualcomm) . Putting these two companies together creates a powerful team geared to successfully solve the challenge of non-invasive diabetic monitoring. The University is interested in the huge impact that a truly non-invasive, wireless wearable device will have on Glucose reading and monitoring, and disease management. ie. Cancer, neurological diseases and chemical dependencies. Given this backdrop, a parnership with a hospital, provider, or device company who is willing to provide needed financing will be key in attaining the success necessary to create a revolutionary working piece of technology that provides the technology to monitor diabetes which has become the worst plagues of our society.
The partnership with a medical provider, physician or medical device company who has the connections and established markets for Aortic blood pressure reading, weight loss, and Kiosk applications would stand be the forefront to a billion dollar industry. Lastly, the University will need funding to complete its work and will work in conjunction with our engineers, and those of Fallbrook Engineering.
We are currently completing the second stage of our prototype development at this stage we are building a chamber the size of a shoe box that will read gasses from the epidermis. The shoebox chamber would be used in Kiosks at Rite Aids and CVS stores. It would replace the current methods of reading blood pressure and give the user a much broader scope of measuring pertainent health and vitality information.
3. We are in private talks with US based Molex Engineering, FoxConn of Tiawan, and Huawei of China. We have garnered major interest from several major companies who is looking for us to develop an aspect of our technology. Yet, we believe the best partnership would be with a device company who already had established markets in our technological space. We are interested in meaningful long-term partnerships that can help channel a technology that will be as revolutionary to the medical device industry as apple was to the computer industry.
Challenge Mission
Market Size
The global CGM market is in its early stages and is rapidly growing. This is mainly due to a rise in the number of diabetics and numerous technological advancements. The CGM market will be valued at $2.9 Billion by 2021 and we believe that with our GDS device, we stand to capture 20% of this market by that time. The standard test strips which represent billions of dollars in spending per year will become obsolete as these CGM devices become more available, giving consumers more information in real time than ever before.Projected 3 Year Growth
We intend to capture 10% of the continuous glucose monitoring market in the next 3 years with at least a 35% gross margin which would equate to over a 150 million dollar market. This number we expect to be conservatively doubled to 300 million dollars during the same period when we add electrolyte detection, and high blood pressure markets, in addition to the professional and amateur sports markets, along with the health and wellness markets, with an average of at least 30% gross margins.How We Will Make Money
Our glucose continuous monitoring technology will be marketed in the following areas: B2B: Kiosks, Diabetic monitoring at hospitals, and wearable technology for diabetes sufferers, we will target hospitals, senior homes, mental wards, Alzheimer centers, Neo-Natal wards, military hospitals & military training, correctional facilities and various other business and organizations. Direct marketing to hospitals (over 5,000 hospitals in USA), senior facilities, home owners, Alzheimer’s Association members, Heart doctors and patients, Obesity, Diabetes, & Stroke patients, Epilepsy, Paraplegic, Quadriplegic, Spinal, Sleep Apnea, Thyroid disease, high blood pressure, Cancer, Parkinson's, Blood clots. and patients at high risk during travel.About our Competition
Competition on the market today are capable of constant glucose monitoring (CGM), but they fall short in accuracy and most are still invasive. Our GDS has the ability to detect blood glucose levels and wirelessly transmit this data to the connected device of the user’s choice. Our device detects the specific signature of glucose emitted from the epidermal layer of skin and is able to measure its exact levels in the blood from there. This can all be done on one device not much bigger than a quarter that is placed directly on the skin. The greatest advantage to the use of this technology is that it can be employed to detect and measure various types of blood content including, but not limited to electrolytes, metabolites, and small molecules such as amino Acids, DHEA and cortisol. We will also be able to detect proteins such as interleukins, tumor necrosis factor, and neuropeptides. We expect this technology to not only enable the creation of devices that will take over the market.Innovation Details
Intellectual Property Summary
Our newest technology has been recently converted from a provisional patent to a utility patent application No.: 14/933985. Our patent attorneys are Knobbe Martens one of the largest most recognized patent firms in the world. We also have 2 provisional patents connected with our foundational patent covering specific areas of technology under development. Additionally, we also have an issued patent on swimming technology US Patent #8,659,435 B2. drowning is the third leading cause of unintentional injury resulting in death. The most effective way to combat this killer is to raise the alarm as soon as a swimmer is experiencing trouble by detecting, reading and transposing the human vitality signals of humans and sending them to a remote location. https://patents.google.com/patent/US20110241887A1/en?q=waterproof&q=optically&q=sensing&q=fiberless
Clinical Information
We plan to have a non-FDA marketed device and a version which will require FDA Class 2 approval. Our sports rendention of our device will detect potassium, magnesium, sodium levels and vitamin D. This will be used in professional and amateur sports. We also are developing a device that will be integrated into cell phone technology. Finally, we have enlisted the help of the University of Arkansas and have a letter of commitment from the University that is available upon request.
Regulatory Status
We have enlisted the services of Fallbrook Engineering in Luecadia to be our contracted design manufacturer and development contractor for medical devices. Since 1981, Fallbrook has been specialized in medical device development with extensive experience in developing monitoring devices for the diabetic market. They have developed medical devices for some of the largest medical device companies in the world including names like Medronics. They also developed one one of the greatest technological developments in stent technology with Scripts and hold a joint patent with Dr. Paul Tierstien who is head of Cardiology at Scripts. They will be assisting us to create the functioning prototype and they have an extensive background in pursuing all FDA approvals.
How we will use the funds raised
Our funds will be used to complete the building of our chamber. The chamber comes with a gasket sealed opening which will allow entry of a human sample for readings in addition to controlled samples and complex customized optic sensors and photospectrometers and other sensing devices that we have developed with our collaborators to complete our chamber. Once fully completed, our chamber will be able to detect Glucose, Electrolytes, Cardio disorders, and much more. Finally funding at this stage will allow us to perform spectral wavelength readings which must be crossed referenced with a database that will reveal the properties of the control sample and display on the front of our chamber. Once we have completed our chamber we will be developing a specialized chamber for the detection and monitoring of cancer and other complex diseases.
Thank You
We need your support, because we will change the world by allowing people to monitor diseases in a way that is easy and efficient. It strongly believe it will save the health care industry billions of dollars. Please consider that none of the devices that fall within our current competition employ the technology of our GDS device, and none will be able to claim the consistency, and accuracy that we will achieve. All of the other technologies need to be calibrated with the traditional invasive blood sugar monitoring. In addition, our technology does not have a ceiling of what other substances can be detected. Our future is not limited to only detecting blood chemicals, but we will be able to impact the body itself by detecting cancer markers or employing gene therapy. Your contribution will go toward making history, you vote is important and valuable in helping to relieve human suffering gobally. Join us!!! We appreciate your support!!!
Updates
-
Update #1
Partnerships
We are open to partners who have technologies that can work in conjunction with our own cutting-edge technology. We also encourage investors who can have an ownership interest Better Life Technologies its patents and areas of patent technology that is still pending. Please let me know of your interest by emailing me at gmckinney@betterlifetech.net
Supporters
-

11/16/2016 - Liked the project.
11/14/2016 - Followed the project. Instant Feedback
Instant Feedback
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.
12Medstartr
Index Score12
Interest
Score0
Adoption
Score2
Likes0
Partners0
Pilots1
Follows-
This campaign has ended but you can still get involved.See options below.
$ 10,000 goal
Instant Feedback
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.

