Assurient: A Health Monitor
by Adam Wilkes

Anticipate. Evaluate. Treat. Early Intervention Means Better Care and Fewer Hospitalizations
Cherry HIll, NJ United States Wearables Medical Device MedStartr Ventures challengeAbout our project
The problem we solve: Most of the $65Bn spent on patients with CHF and COPD is for inpatient care. Many of these admissions would have been avoided if treatment had been started earlier. Comprehensive disease management programs are costly and would be far more effective if they targeted the patients who are at highest risk
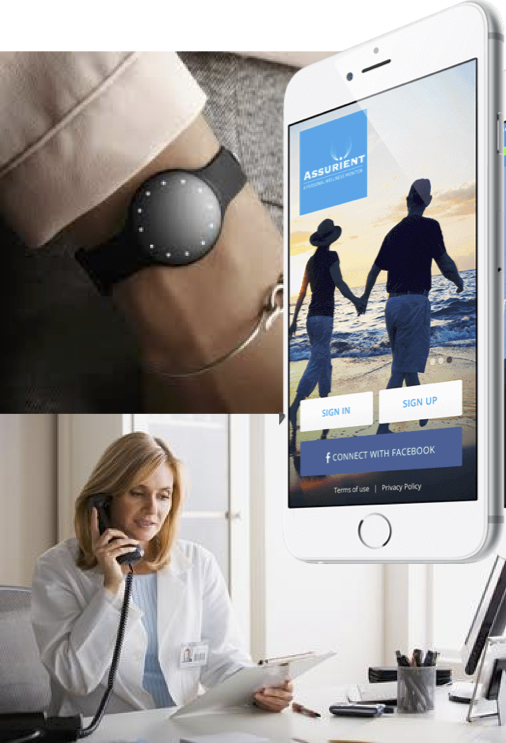
About our solution: Assurient leverages the observation that as patients with chronic diseases begin to decompensate they alter their behavior and physiology in predictable ways which are detectable by a wrist-worm monitor. The monitor collects data which is transferred to our server via smart phone for analysis. When Assurient detects the 'footprint' of an early, acute exacerbation, a report is sent to the interested stakeholders for early intervention.
Progress to date:
We have been very well received in the marketplace. We will be conducting a pilot study with Aledade ACO in the Philidelphia suburbs in March, 2017
About Our Team
Creator: Adam Wilkes
Location: New Jersey
Education: Thomas Jefferson University
Bio: Adam has served at as a senior healthcare consultant for PwC, a Medical executive with Premier Urgent Care, and a physician and founding partner of a large ER group. He lead the team that developed iLabsDDx, the iPhone’s fastest growing medical calculator. He is a board-certified Internist with 25 years of clinical experience during which time he has cared for over 75,000 patients.
Title: Founder
Advanced Degree(s): M.D.
About Team Members
Daniel Endy
Business Strategy, Boston University BS CE, Computer Engineering, Software, Physics
Biography: Daniel has extensive experience as a Strategic Consultant and as an Advisor to Startups and New Product Groups. He several successful exits including co-founding US Interactive (IPO), and Coates Analytics (Acquired by PNC Bank), advising Boomi (Acquired by Dell), and investing in Five Below (IPO).
Title: Business Strategy
Advanced Degree(s): Boston University BS CE, Computer Engineering, Software, Physics
LinkedIn:
https://www.linkedin.com/in/danielendy
Andrew Rothman
Biostatistician, Harvard University Master of Science (M.S.), MIT PhD candidate
Biography: Andrew is a Harvard & M.I.T. trained Data Scientist, Statistician, and Machine Learning Expert with 7+ years experience including working with Healthicity, Experfy, and Booz Allen Hamilton
Title: Biostatistician
Advanced Degree(s): Harvard University Master of Science (M.S.), MIT PhD candidate
LinkedIn:
https://www.linkedin.com/in/andrew-rothman-49739630
About Our Company

Assurient
Location: 411 Society Hill Blvd
Cherry HIll, NJ 08003
US
Founded: 2016
Product Stage: Prototype/MVP
YTD Sales: Working on it
Employees: 3-5
How We Help Patients
Assurient directly addresses the triple-aim of improving the patient experience, improving quality, and controlling costs.
- Assurient is an attractive and unobrusive monitor whose direct benefit to the patient is to keep them healthy and out of the hospital
- Assurient functions as a screening test, identifying patients at-risk for hospitalization. By evaluating patients at this early stage and avoiding an inpatient stay, patients are spared significant moribidy and iatrogenic risks
- There is a 100x diffence in cost between inpatient and outpatient care for CHF and COPD.
How We Help Physicians
As a physician, how often had you wished that you had had the opportunity to treat your patient in the days before she presented to the ER requiring inturbation? How many hospital admissions could have been avoided with a timely diuretic adjustment, or the addition of a steroid?
Assurient is the solution to the problem. By monitoring patients for the early signs of the acute decompensation, Assurient reports which patients should be seen today in order to avoid an ER visit and hospitalization.
How We Help Hospitals
In 2017, CMS will withhold more $540 million from over half of the nations hosptials as readmission penalties (Hospital Readmission Reduction Program (HRRP)). ACOs too have a significant financial incentives, as do insurance providers.
Assurient provides a tool which can help avoid these penalties.
Arrurient is a wrist-worn device that monitors recently discharged patients. Patients who are progressing appropriately can be triaged to usual care; patients who trajectory appears stagnant can be targetted for much more aggressive care, and those whose condition appear to be worsening can be referred for immediate evaluation by thier primary providers.
Assurient only needs to help prevent 5% of admissions to realize a positive ROI for hospitals and 3rd party payers
How We Help Partners
Device Manufacturers:
All fitness trackers appeal to the same market segment; younger and health-conscious people who are looking to track their progress or for motivation to improve their health. Assurient expands that addressable market by giving 50+ million elderly Americans and thier adult children a reason to purchase a fitness tracker.
Challenge Mission
Key Milestones Achieved and Planned
We intend to achieve 3% market penetration within 3 years which will result in 480,000 users, producing $200M in revenue in 2020.
By 2020, we anticipate that continued improvements in battery technology will make it practical to include Galvanic Skin Response and other physiologic parameters into our algorithm. This will allow us to include episodic infection and ischemic heart disease as part of our service offering, expanding the total addressable market to 50M Americans.
Our Competitive Advantages
While Assurient brings value to each of the four main stakeholders in heathcare (patients, providers, partners, and institutions), we believe that our greatest opportunity is to directly address the Divisions of Innovation and Disease Management Departments within 3rd party payers.
- The pressures of Value Based Purchasing and the necessity of cost containment will make Assurient very attractive to them
- This approach allows us to leverage several hundred companies to access millions of users
- 3rd party payers are skilled at encouraging patient compliance
- That Assurient will originate from thier own insurance company will give it immediate credibility
At a price point of $24/user/month (which is 62% margin), the 3rd party payer breaks even by avoiding only 6% of admissions. At the predicted 15% of admissions avoided, their ROI is about 100%.
Barriers to Entry
The development of this application for wearable technology was inevitable. Currently there are several companies that use monitors to detect deviations from the user’s baseline (QMedic, CarePredict, HealthSense, Lively, and others).
Assurient differentiates itself by:
- Assurient monitors the user as opposed to the environment. This confers the attributes of being continuous without being intrusive
- Assurient's algorithms are built on raw accelerometer data, on a scalable platform which can accomodate additional physiologic parameters as they become practical to use
- Assurient uses a monitoring system which is attractive and has a 6+ month battery life compared to the 5-7 days of the other devices. This is of paramount importance in this population for patient comliance
- While America’s largest companies (Microsoft, Google, Apple) are also exploring big data and its relationship to health and disease (Apple Healthkit and ResearchKit, Google Fit, and Microsoft Health) and the basic science literature is beginning to report the ability of wearable biosensors to reveal useful health information, none of these large companies has yet applied it to patient care. Assurient will benefit from first-mover advantage in securing relationships with key 3rd party payers
Traction, Funding and Partners
In dozens of informal discussions, Assurient has been universally received positively.
An anecdote. In seeking a partner with whom to conduct a pilot study we used an aphabetical list of ACO's. The first call was to Aledade, who is enthusastic to do the pilot, beginning in March. We didn't need to make a 2nd call.
Innovation Details
Intellectual Property Summary
We have applied for and been granted a provisional patent.
Currently we are holding our IP as a trade secret; but will ultimately be seeking the protection of a nonprovisional patent.
Clinical Information
There is a large body of literature that lead us to develop our algorithm, some of which I site below.
The pilot study in March will be the first direct proof of efficacy.
- Schiff, Gordon D., et al. "Decompensated heart failure: symptoms, patterns of onset, and contributing factors." The American journal of medicine 114.8 (2003): 625-630.
- Jehn, Melissa, et al. "Association of physical activity and prognostic parameters in elderly patients with heart failure." Journal of aging and physical activity 19.1 (2011): 1-15.
- Chaudhry S, et al "Risk factors for hospital admission among older persons with newly diagnosed heart failure findings from the cardiovascular health study" J Am Coll Cardiol 2013; 61: 635-642.
- Passantino, Andrea, et al. "Short-term change in distance walked in 6 min is an indicator of outcome in patients with chronic heart failure in clinical practice." Journal of the American College of Cardiology 48.1 (2006): 99-105.
- Hanly PJ, Zuberi-Khokhar N. Periodic limb movements during sleep in patients with congestive heart failure. Chest. 1996;109(6):1497–502
- Hanly, Patrick, and Naheed Zuberi-Khokhar. "Daytime sleepiness in patients with congestive heart failure and Cheyne-Stokes respiration." CHEST Journal 107.4 (1995): 952-958.
- Redeker NS. Sleep disturbance in people with heart failure: implications for self- care. J Cardiovasc Nurs. 2008;23(3):231-238.
- Redeker NS, Jeon S, Muench U, Campbell D, Walsleben J, Rapoport D. Insomnia symptoms and daytime function in stable heart failure. Sleep. 2010;33.
- Erickson VS, Westlake CA, Dracup KA, Woo M, Hage A. Sleep disturbance symptoms in patients with heart failure. AACN Clinical Issues. 2003;14(4):477-487.
- Brostrom A, Stromberg A, Dalstrom U, Fridlund B. Sleep difficulties, daytime sleepiness, and health-related quality of life in patients with chronic heart failure. J Cardiovasc Nurs. 2004;19(4):234-242.
- Gheorghiade M, De LL, Fonarow GC, et al: Pathophysiologic targets in the early phase of acute heart failure syndromes. Am J Cardiol 2005; 96:11G–17G
- Emerman CL: Treatment of the acute de- compensation of heart failure: Efficacy and pharmacoeconomics of early initiation of therapy in the emergency department. Rev Cardiovasc Med 2003; 4(Suppl 7):S13–S20
- van den Berg-Emons, Hendrika JG, et al. "Validity of ambulatory accelerometry to quantify physical activity in heart failure." Scandinavian journal of rehabilitation medicine 32.4 (2000): 187-192.
- Jehn, Melissa, et al. "Accelerometer-based quantification of 6-minute walk test performance in patients with chronic heart failure: applicability in telemedicine." Journal of cardiac failure 15.4 (2009): 334-340.
- de Zambotti, Massimiliano, et al. "Evaluation of a consumer fitness-tracking device to assess sleep in adults." Chronobiology international 32.7 (2015): 1024-1028.
Regulatory Status
An FDA Pre-Submission Review is pending. Assurient will likely follow the 510(k) pathway since other similar monitors such as the ActiGraph have received this clearance.
How we will use the funds raised
IT:
- Infrastructure Development
- Continue to refine algorithm
- Continued Software development
Business Uses:
- Sales & marketing
- Legal
Thank You
Thank you for your interest and I hope you join us as we bring Assurient ito market. It is an elegant monitoring system that will make an impact on the health of our users while containing costs.
Updates
No updates found .
Supporters
-
 , MBA
, MBA
11/23/2017 - Followed the project. , MBA
, MBA
11/23/2017 - Liked the project. , BA
, BA
11/13/2017 - Liked the project., Master’s Degree in Business Administration from Seton Hall University
10/06/2017 - Liked the project., Master’s Degree in Business Administration from Seton Hall University
10/06/2017 - Followed the project.
02/28/2017 - Followed the project.
02/28/2017 - Liked the project.
02/28/2017 - Followed the project.
02/28/2017 - Liked the project.
02/28/2017 - Liked the project.
02/28/2017 - Followed the project.
02/26/2017 - Liked the project.
02/25/2017 - Liked the project. , BA
, BA
02/24/2017 - Followed the project.
02/24/2017 - Liked the project.
02/24/2017 - Followed the project. , MS
, MS
02/10/2017 - Interested in helping your project as a mentor or team member. Instant Feedback
Instant Feedback
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.
25Medstartr
Index Score25
Interest
Score0
Adoption
Score10
Likes0
Partners0
Pilots8
Follows-
This campaign has ended but you can still get involved.See options below.
$ 20,000 goal
Instant Feedback
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.