Diagnosis of pre-eclampsia: It's all about Potassium
Making pre-eclampsia a disease rather than a syndrome
Belmont, CA United States Diagnostics Women(s) Health Cardiovascular Disease 2021 Vision challengeAbout our project
The problem we solve: Pre-eclampsia is the single largest cause of morbidity and mortality during pregnancy, effecting 3-5% of women in the USA. The symptoms of pre-eclampsia are new-onset of both hypertension and proteinuria by 20 weeks of gestation. After parturition, there is a 3-4 fold increased risk of high blood pressure and double the risk of heart disease and stroke for both the affected infant and the mother. There is also a 4-6 fold increased risk of end-stage renal failure in the affected woman. The biochemistry of the enhanced risk for these health problems is completely unknown. At present, there is no FDA approved diagnostic marker for pre-eclampsia or method to monitor progression. Without an assay for diagnosis or progression, no therapeutic intervention has been developed and approved by the FDA, other than immediate delivery of the fetus, if (when) the increased blood pressure becomes life threatening.
About our solution: In 2018, IOMA discovered spiral steroid phosphoesters. Initially, these compounds were isolated by monitoring cross-reacting material (CRM) with digoxin specific antibodies. We now know that CRM are phosphocholine steroid esters with 23 or more carbon atoms. Each of the 8 CRM we identified has a spiral lactone E-ring, which resembles synthetic potassium diuretics, such as spironolactone. Our logo shows a sample E-ring. Although an endogenous mammalian potassium sparing hormone has not previously been described, IOMA’s solution is based on the proposed role of these compounds during pregnancy. In 2019, IOMA completed a pilot study indicating that women with pre-eclampsia have elevated levels of C313 and/or K475. IOMA proposes to satisfy the unmet need by measuring serum levels of these individual steroid phosphocholine esters precursors as a screening test for risk of pre-eclampsia. A follow up MS test will be used to confirm the diagnosis.

Progress to date:
- Isolation of 6 phosphocholine (PC) steroid esters from human serum. 5 of these have 23 carbon atoms. Of these, 3 are both DLM and spiral lactones and 2 are carboxylic acids. The 6th is a precursor with 21 carbon atoms.
- We are currently investigating: [a] pathway for biosynthesis, [b] function, [c] endocrinology and [d] pathology.
- Pediatric pilot study (n=40) with LC-MS assay prototype. Patients: children with thyroid disorders, diabetes, or GH deficiency. Result: PC-steroid ester pattern not altered. No evidence for episodic secretion.
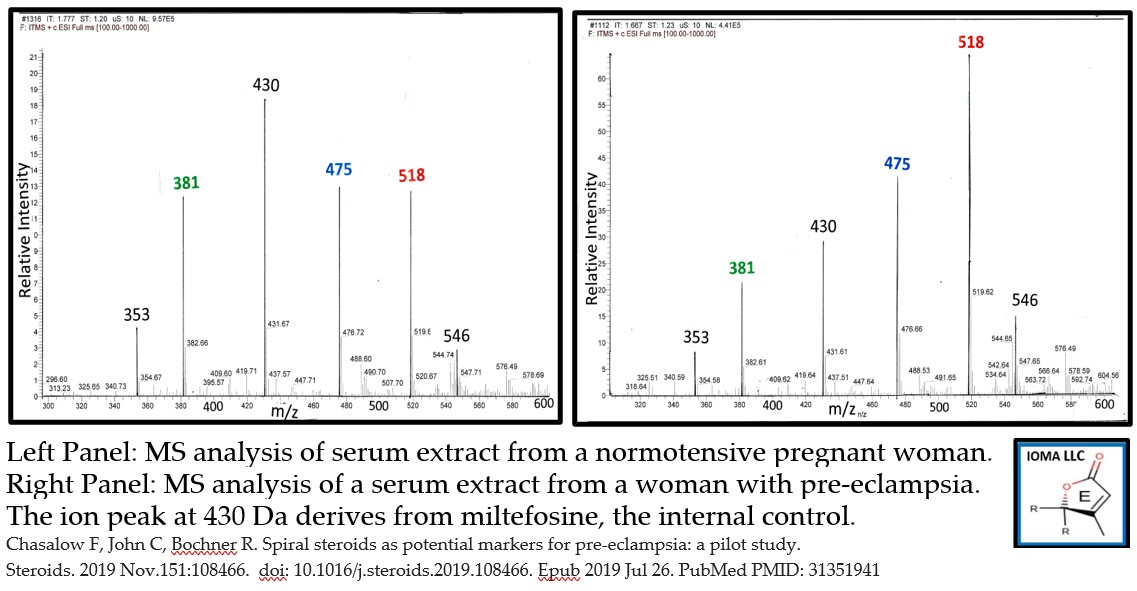
- Pre-eclampsia pilot study (n=40) with MS-MS assay prototype. Patients: pregnant women-24-26 weeks of gestational age; normotensive (n=20), preeclamptic (n=20). Z score based on ion intensity of the normotensive women. Result: 1 of 20 normotensive women had a Z-score over 2. 12 of 20 women with pre-eclampsia had a Z-score over 2 for at either C313 or K475. The frequency is statistically significant at the P<0.05 level. See attached graph
- PrePrint. doi:10.20944/preprints202007.0211.v1 Phosphocholine Steroid Conjugates: Are These Compounds the Mammalian Cardiotonic Steroids. Fred Chasalow.
About Our Team

Creator: Fred Chasalow
Location: California
Education: Ph.D. Biochemistry, Brandeis University
Bio: I studied in leading institutions and I was mentored by leading scientists. Dr. Kaplan wrote and edited Methods in Enzymology. Dr. Pharriss was a leading scientist at Upjohn for the discovery of prostaglandins. Dr. Lieberman was a leading steroid investigator including the first use of a steroid as hapten to generate specific antibodies. Dr. Wall discovered taxol and vincristine. In 1981, I started my academic career. At the time, the commercial laboratories were designed for adult samples. My laboratory developed sensitive and specific assay methodology that was applicable to pediatric samples. It was during this period that my team recognized that Smith-Lemli-Opitz Syndrome (SLOS) was a steroid biosynthetic defect. In 1991, I was promoted to Professor of Pediatrics, Director of Pediatric Research and Chief of Pediatric Endocrine Laboratory at the Maimonides Medical Center of SUNY Brooklyn. My laboratory research activities were funded by my clinical laboratory service, but I also had grants from NIH, March of Dimes, and State of New York. I was the first Ph.D. member of the Lawson Wilkins Pediatric Endocrine Society. In 1992, Dr. Leon Bradlow and I co-founded AMUR Research. Wilson Sonsimi was our advisor. The commercial premise was that the hormone missing in patients with SLO syndrome might be a useful drug. In 1995, AMUR opened a laboratory at the Incubator at Stony Brook, NY and I committed to working full-time as CEO and Chief Scientific Officer (CSO). At AMUR, I confirmed that the inborn error in patients with SLOS led to the absence of a novel steroid phosphocholine ester. I was personally responsible for 6 patents. In 2015, I developed a trial and error approach and determined the chemical formula and structure of the endogenous steroid phosphocholine ester. I established IOMA LLC. At present, I function as the Managing Partner and CSO and I am the sole (unpaid) employee.
Hospital Affiliation: Visiting Professor, Dept of Laboratory Medicine
Title: Managing Partner, Chief Scientist
Advanced Degree(s): B.S. Unified Science, Stevens Institute of Technology
About Team Members
Ron Bochner
Chief Medical Officer, M.D.
Biography: Education: Mt. Sinai School of Medicine class of 1981, completed residency training in obstetrics and gynecology at The Long Island Jewish Medical Center.
Academic career: Instructor at the Robert Woodward Johnson Medical School in New Brunswick, New Jersey from 1987 to 1988. Appointed as clinical associate professor in 1992. In January 2021, Bochner is slated to be president of the medical staff with responsibility for 3000 physicians.
Clinical Career: Entered private practice in 1988. Established a medical practice which now has seven physicians and delivers 1000 babies per year. Service and care is provided for a full range of both obstetrical and gynecologic conditions. Obstetrical experience encompasses the delivery of 6000 babies over 35 years and hundreds of surgical procedures both major and minor.
Scientific Activities: Co-investigator with Fred Chasalow investigating a novel steroid phosphocholine ester named Ionotropin. Measurement of serum levels of Ionotropin may have utility for the early detection of pre-eclampsia and monitoring progression to eclampsia.
Entrepreneurial Activities: Founded Retrofit LLC, a medical device company holding several patents of his own design, one of which (Gynocart) is currently in 1100 emergency rooms in the USA. Inventor of “Rapid Deployment Stirrups” a military medical device currently being evaluated by the Military medical branch. Inventor of D-Helmet a foldable bike helmet that will be utilized in the bicycle/scooter rental market.
Title: Chief Medical Officer
Advanced Degree(s): M.D.
About Our Company

IOMA LLC
Location: 2523 Hastings Drive
Suite D
Belmont, CA 94002
US
Founded: 2015
Product Stage: Prototype/MVP
Employees: 1-2
How We Help Patients
Syndrome: A number of symptoms often occuring together but without a known cause. Disease: A particular distinctive process in the body with a specific cause and characteristic symptoms. It is not uncommon to have two syndromes with different biochemical causes. Perhaps diabetes is the most well-known example. Patients with both Type 1 and Type 2 diabetes have the same symptom - high blood glucose levels. However, Type 1 patients don't synthesize insulin and insulin doesn't workin Type 2 patients. In summary, this is an example showing one syndrome can have multiple diseases.
Pre-eclampsia is a syndrome diagnosed during the 2nd half of pregnancy. It is based on the characteristic symptoms of high blood pressure and proteinuria (unexplained high concentrations of proteins in the urine). It is a syndrome rather than a disease because the specific cause is unknown. These is no FDA approved diagnostic or therapeutic protocol. About 5-8 % of women develop pre-eclampsia during the second half of gestation. Without a diagnosis, this is very unsettling for the affected women. Worse, 5% of the women with pre-eclampsia develop life-threatening hypertension. Thus, the doctor has to say he doesn't know the cause and he doesn't have a therapy. All the doctor can say or do, is that he will see her regularly and, if the hypertension becomes life-threatening, he will deliver the infant by C-section. This is the occasion the doctor asks his question, "If I can only save you or the infant, which one should I save?" As a side note, three of my acquaintences were asked that question.
Our theory is that pre-eclampsia is actually two diseases. In the video, one disease was described as Class C and the other one was decribed as Class E. What makes them a disease is the levels of the phosphosteroid precursors: Class C - has high serum levels of C313 or K475 (11-hydroxy-C313). Measurement of the phosphosteroid precursors could be useful as a marker to guide physicians in therapeutic choices and protocols. Class E - has normal serum levels of the phosphosteroids we measured. Class E has a different underlying cause, for which we have no theory. Without the high levels of the precursors, the patients should be at low risk of developing life-threatening high blood pressure. This prediction needs to be confirmed.
Class C pre-eclampsia will become a treatable disease. Physicians will be able to monitor disease progression and to develop appropriate therapeutic regimes. Every year 50,000 women experience life-threatening events during pregnancy. Women are screened for gestational diabetes and thyroid disorders, but there is no FDA approved method for screening for gestational electrolyte disorders. With our discovery of the spiral steroids that now becomes possible. In brief, having a diagnostic makes all of the difference.
How We Help Physicians
Doctors do their best to care for their patients. However, pre-eclampsia is best described as a syndrome characterized by high blood pressure and proteinuria. Neither symptom is easy to quantitate and neither is a useful marker to monitor therapy. During the second trimester, symptoms develop but the underlying, biochemical basis is unknown.
We think we have the solution.
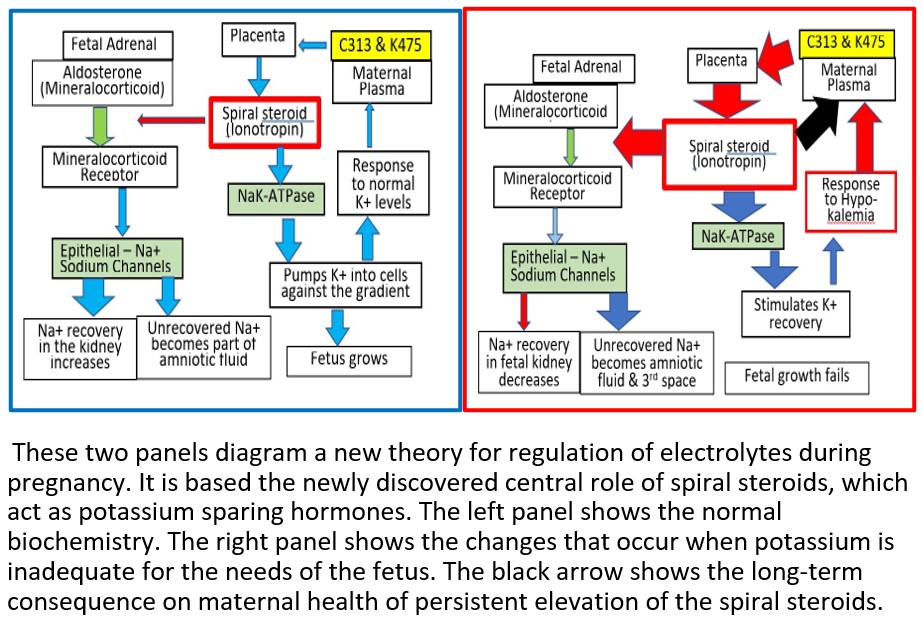
Our theory is that the underlying cause of pre-eclampsia is potassium deficiency in the fetus. In response, the mother secretes a spiral steroid precursor (C313 and/or K475). In the placenta, the precursor is converted to a spiral steroid which functions as a potassium sparing hormone (C341). For most patients with pre-eclampsia, this process is sufficient to provide the potassium needed by the fetus. However, in 5-10% of patients with pre-eclampsia, apparently, the regulatory path fails. The fetal-placental unit overcompensates by secreting excess C341, some of which diffuses into the maternal circulation and causes maternal toxicity that resembles digoxin toxicity. It can even be treated with digibind. It has long been known that, during the third trimester, an unknown digoxin like material (DLM) can be detected in serum from patients with pre-eclampsia. However, its identity was unknown. Now we know that, at least some DLM, are phosphocholine esters of spiral steroids. One example is C341.
All together, we have identified more than 6 spiral steroids that can be detected as DLM. This is probably the basis for the fact that DLM levels do not correlate with disease status.
We think physicians will seek out our assay to help guide their treatment of patients with pre-eclampsia and are at risk for life-threatening hypertension. We need your help.
We need your investment to fund the laboratory operation.
We need your help in gathering samples for analysis.
How We Help Partners
If you are looking at this page, you have probably made your money and had a successful career. Thirty years from now, will anyone remember your name and what you did? Just thinking: here is a list of the discoverers of the steroids:
Time line for discovery of steroids
Year Discovery Function Discoverer Steroid class
1935 Testosterone Androgen E. Laqueur 19 Carbon
1934 Progesterone Progestogen A. Butenandt 21 Carbon
1929 Cortisone Glucocorticoid E. Kendall & H. Mason 21 Carbon
1929 Estrone Estrogen A. Butenandt & E.A. Doisy 18 Carbon
1959 DHEA-sulfate Weak androgen E. Baulieu 19 Carbon
1954 Aldosterone Mineralocorticoid S. Simpson & J. Tait 21 Carbon
When I was a student; these were my heros; a few were still alive. Even if you don’t know their names, you do know their discoveries and you know the impact they have had on medical care. Here is the latest entry on the list:
2018 Ionotropin (C341) Anti-mineralocorticoid F. Chasalow & L. Pierce-Cohen 23 Carbon
By partnering with IOMA, you have the opportunity to become part of the history of science and make money doing it. Is that something which you would like to do?
The first application of the discovery is a diagnostic for pre-eclampsia. That is the central theme of this proposal. We also ‘know’ that the assay will have applications to many other diseases: [a] salt-sensitive hypertension; [b] congestive heart failure; [c] atrial fibrillation, [d] polycystic kidney and/or ovary disease and [e] PTSD. In some diseases, it is a deficiency and in some it is a surplus. Mass spectroscopy has detected 3 different compounds distinguished by the number of carbon atoms and there are also multiple forms based on different oxygen atoms. This plethora of compounds, most of which are detectable by cross reaction with antibodies to ouabain or digoxin, is probably the key reason so little is known about the existence or function of the individual hormone families.
For each indication, we need to ‘tweak’ the assay and organize a clinical trial. There is a role for partners for every indication and probably for other diseases that are unknown to me. Ultimately, we will identify which spiral steroid is deficient in a particular disease and synthesize the desired compound. Replacement hormone therapy would be a direct path to a huge payoff.
Right now, we are limited in three ways: [1] our lab is closed because of the potential for covid 19 infection; [2] lack of money and skilled scientists for the laboratory; and [3] a leader to organize clinical trials and the funds for their execution. Problem [1] will go away with the validation of a vaccine or other means of reducing infection. Problems [2] and [3] need skilled individuals and financial partners.
Would you like to join our Team? What position would you like to play? Send me a proposal and lets talk.
Challenge Mission
Innovation Details
Intellectual Property Summary
The compounds we isolated are natural products. Composition of matter patents are not allowed. Given that statement, ‘use’ patents are allowed. The circumstances have been reviewed by my patent attorney, Dr. Howard Frankfort. He is well-versed in this project and has already written 6 patents for me. He believes IP is possible and is prepared to start work when IOMA can sign a retainer. Pre-eclampsia is considered an orphan disease. There is also protection for Intellectual Property in the FDA approval process under the orphan disease provisions.
Patent Link
Not Available
Clinical Information
These is no FDA approved method of diagnosis for pre-eclampsia. There is no recognized therapeutic approach. The only therapy is delivery of the fetus when the maternal hypertension becomes life threatening. Physicians are often put into a quandry: save the mother by delivering the infant or save the infant from a premature delivery at the risk of maternal strokes due to extreme hypertension.
During pregnancy, fetal nutrition comes through the umbilical cord. The electrolytes in the umbilical cord are 145 mM Na+ and 4-6mM K+. Fetal tissues are approximately 100 mM K+ and 10 mM Na+. Thus, there must be a mechanism to concentrate the K+ against the gradient. As gestation progresses, there is increased need for K+ for the fetal tissue mass and blood pressure must increase due to the increased length of blood vessels. At parturition, the situation changes. Nutrition is no longer provided via the umbilical cord but by mother’s milk. Milk is high in K+ and low in Na+. During the first week, newborns are Na+ wasting and lose about 10% of their body mass. By the end of the 2nd week, Na+ wasting has stopped and growth resumes. The biological sequence has been known but the underlying biochemistry was not known. (doi: 10.31080/ecpe.2018.07.00267)
Our theory is:
- These observations are the direct result of the function of an endogenous K+ sparing hormone.
- That one or more of the phosphocholine spiral steroid esters that we have just discovered are the endogenous K+ sparing hormone. (doi: 10.1016/j.steroids.2018.03.001).
- The endogenous spiral steroid regulates NaK-ATPase, perhaps like spironolactone, with which it shares structural features.
IOMA has just completed a pilot study. Serum levels in normotensive pregnant women (n=20) were used to establish normal values which were used to generate z-scores in serum from women with pre-eclampsia (n=20). In the normotensive group, only one sample had a value with a z-score over 2. In contrast, 12 samples from women with pre-eclampsia had at least one of the phosphocholine steroid hormones with a Z-score over 2. This difference is significant at the P<0.05 level. (doi: 10.1016/j.steroids.2019.108466)
There seemed to be two populations – Group C with elevated levels of either K475 or C313 and Group E with no elevated levels of any phosphocholine steroid in the maternal circulation. We propose that this observation suggests that there are two separate pathological processes that can lead to pre-eclampsia. A follow up study is necessary to confirm which group develops life-threatening hypertension.
There were several useful conclusions from this study:
- Pre-eclampsia seems to be two disorders with similar symptoms. Clinical investigations would be confused by the lack of a common disorder. This might be a contributing factor to the lack of an FDA approved therapy. Clinical trials will have to consider this observation in their design.
- Potentially, one of the two groups will not be at risk for life-threatening hypertension. The women in this group would be thrilled, if that is the outcome.
- Potentially, patients with life-threatening hypertension might have persistent high levels of spiral steroids after parturition. This might be the basis for the long-term continuing risk of cardiovascular and renal consequences. Similar changes are observed when cardiotonic glycosides are administered to animal models of pre-eclampsia.
Regulatory Status
Our diagnostic test for pre-eclampsia will qualify as a Laboratory Developed Test (LDT). An LDT is an IVD (In vitro diagnostic device) “that is intended for clinical use and designed and manufactured within a single laboratory.” Potential competitors cannot use our method or reagents but must develop their own method and perform their own clinical trials to establish normal ranges for their test.
Pre-eclampsia has orphan disease status. Potentially, this provides a 7-year period of no competition and qualification for the Humanitarium Device Exemption.
How we will use the funds raised
1. Corporate organization $25,000
Making IOMA LLC a corporation, including:
D&O insurance; Board of Directors; Compliance with corporate law
2. Consultants $25,000
Chief Financial officer; FDA regulatory expert; CLIA expert; IRB expert; CPT code expert
3. Acquisition costs for samples $30,000
Serum – GAPPS possible supplier; From women with life threatening hypertension
Post parturition – post eclampsia, Cord serum
Placenta - IIMC possible supplier
Animal tissues
4. Contracts for production of antibodies and for NCIRE $40,000
5. Laboratory supplies $60,000
Solvents, glassware, disposables - 30,000; Annual service for Mass Spectrometer - $20,000;
Preparative columns - $10,000
6. Salaries : Research Associate $ 90,000
7. Sub total $270,000
Fee for “keep what you get” $ 30,000
8. Proposed budget $ 300,000
Notes
It is not my intention to accept a salary during the period covered by this budget. All donations will be used [a] to develop the assay for the phosphosteroids and their precursors, [b] to evaluate the assays during pregnancy, pre-eclampsia and other diseases of pregnancy. and [c] to prepare the corporate structure for the next round of funding. I expect to have a salary after this period.
Thank You
The basis for this project is our discovery of the endogenous potassium sparing hormone. The discovery includes both an unexpected family of steroid esters and a hormone with an unexpected function.
For hundreds of years, physician-scientists have sought the explanation for the cause of high blood pressure during pregnancy. Based on our discovery, we believe we are close to finding the pathology. Our theory may also account for the increased long-term risk for cardiovascular and kidney dysfunction. Our team needs two kinds of help. We need [a] willing donors of money and [b] serum from women who did have, or are now experiencing, high blood pressure (hypertension) during pregnancy.
If we are correct, physicians will not need to ask, if I can only save you or your baby, which should I do?
Please help me - Help you
Updates
No updates found .
Supporters
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.
Index Score
1
Score
0
Score
1
Likes0
Partners0
Pilots0
Follows-
This campaign has ended but you can still get involved.See options below.
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.