GlucoseMama Kits: The first all-in-one kit for diabetes during pregnancy
by Catherine Cheremeteff Jones

The GlucoseMama Kit is a one-box solution to self-managing diabetes during pregnancy. It’s got everything our patients need - plus a whole lot of love!
Bethesda, United States Diabetes Women(s) Health The Diabetes challenge NOLAHI Challenge - See AllAbout our project
The problem we solve: Rates of gestational diabetes mellitus (GDM) are growing exponentially every year. There is an urgent need for an effective and cost-saving self-management system for pregnant patients with diabetes. Currently, the majority of patients are logging daily blood glucose levels with pen and paper. This is inadequate. It offers no immediate feedback, support, or educational tools - and no way to share data with doctors in real time. Paper logs frequently get lost or women forget to bring them to appointments. Many GDM patients are given only one nutritional counseling session. After that they are on their own. Patients are anxious and frustrated by the daily grind of self-management. Doctors and care teams need a better way to receive data to treat their high-risk patients.
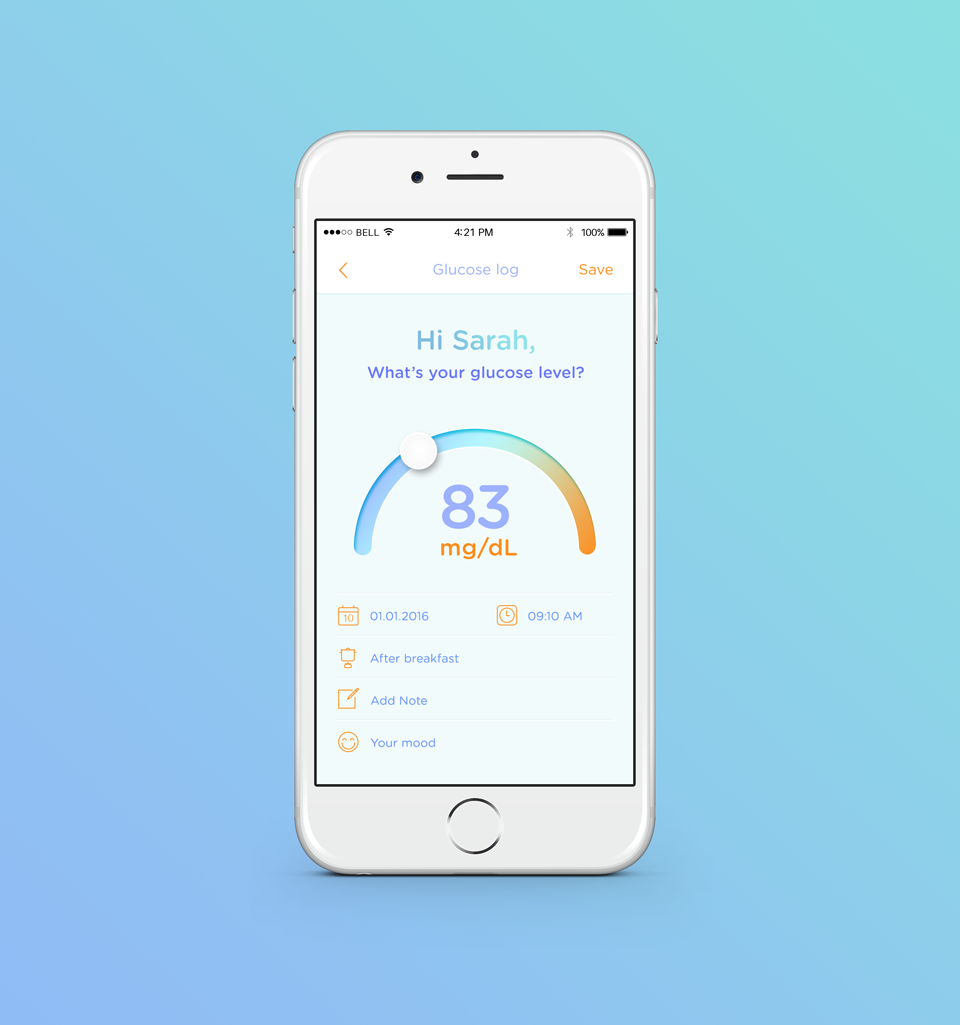
About our solution: After talking with dozens of patients, doctors and clinical teams, we created the GlucoseMama Kit. Our all-in-one self-management platform includes both hardware (glucometer, strips, lancing device and lancets) and HIPAA-compliant software. Doctors tell us they want to write one script for everything (we're working on this). Patients tell us they need tools to help them count carbs, make smart food choices, exercise, and stay on track every day. Both groups tell us they want a secure form of communication to share data, guide treatment, and deliver healthy babies. Our platform answers all of these needs and more.
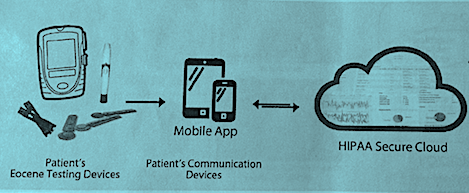
Progress to date:
Our team has made tremendous progress in two years. Our biggest achievement was recieving funding for a clinical trial with the University of Maryland Medical Center Maternal Fetal Medicine Department. We have a prototype of the GlucoseMama Kit scheduled for a pilot test in March 2018 with the Maternal Fetal Medicine Associates of Maryland. We are reaching out to hospitals and healthcare systems for more pilots. The response from the medical community has been overwhelmingly positive. Patients and doctors love our system. Compliance with blood glucose logging among our beta testers is extremely high. One of the many quotes from our beta testers reads: “I really like everything about it. Overall, I like the feel of the app. Communication between patient and MD is very important to me.” And, one of the MFM specialists we work with said she would like to see the GlucoseMama Kit become the standard of care. That’s our goal, too!
About Our Team

Creator: Catherine Cheremeteff Jones
Location: Maryland
Bio: Catherine Jones is the co-founder and CEO of Werbie, LLC, a startup dedicated to building solutions for women with diabetes. Jones and her software development team, along with Werbie's in-house medical experts, created the GlucoseMama Kit to help pregnant women self-manage diabetes during pregnancy and securely share logging and other data with care teams. GlucoseMama is currently in a clinical trail with University of Maryland Medical Center in Baltimore, funded by the Maryland industrial Partnership (MIPS). Jones and her co-founder, Dr Teresa Knight, MD, OBGYN FACOG have a strong and deep commitment to women's health and wellness. Jones's career as an award-winning author of nutrition cookbooks began with Eating for Pregnancy: The Essential Nutrition Guide and Cookbook for Today’s Mothers-to-Be. She is currently working on the 3rd edition to celebrate the book's 15 years in print.
Title: Co-Founder and CEO
About Team Members
Teresa Knight
Dr, MD OBGYN FACOG
Biography: Dr. Teresa Knight, OBGYN, FACOG is the co-founder of Werbie. She has been the CEO of Women's Health Specialists of St.Louis for the past 14 years. She is passionate about women's health both on a national and international level. She is also a consultant in the health tech space. She is a frequent guest on national TV, and has traveled the globe to promote women's health and wellness through lectures, symposiums, and medical missions. Currently, she has a monthly interview on a local television program called Great Day Saint Louis. She also lectures to resident physicians, consults for companies, and works internationally to help solve myriad challenges in the field of women's health.
Title: Dr
Advanced Degree(s): MD OBGYN FACOG
Twitter:
@WerbieLLC
LinkedIn:
https://www.linkedin.com/in/teresa-knight-8487b932/
About Our Company

Werbie, LLC
Location: 4803 Dover Court
Bethesda, 20816
US
Founded: 2014
Website: http://www.werbie.co
Twitter: @WerbieLLC
Facebook: https://www.facebook.com/werbieapps/
Product Stage: Ready
YTD Sales: Working on it
Employees: 1-2
How We Help Patients
Hi patients! We love you! We are 150% focused on YOU because by helping you succeed, doctors, care teams, hospitals, and insurers succeed. You drive the data for your care teams. We know that pregnancy is hard enough without diabetes. We're proud of the feedback from beta testers. “I am eating less carbs since I started using the system. The text messages remind me that I’m eating for my baby. I won’t have the cookie.” and “It was really helpful for me. Because you know someone is reinforcing what you’re doing is something important. The text messages encouraged me to log every time and giving someone my report felt like I was not only doing this for myself. By sharing my report, I can have feedback like I’m doing bad or good.”
How We Help Physicians
Hi Physicians and Providers! Thank you. Thank you. Thank you. You are our heroes! You manage these high-risk cases and deliver positive outcomes everyday. Catherine Jones, CEO, had two high-risk pregnancies so she knows exactly how much work you do. She's a "NICU Mom" too, so she understands the incredible stress parents face when serious complications arise. What we hear from our in-house medical team and providers is that you need patient data in a quick-and-easy-to-digest format sent to you automatically every week or two, and on demand. We would love to partner with your clinics, hospitals and healthcare systems.
How We Help Hospitals
Hey there, hospitals! We hear your pain points over and over again and we want to help you. Your awesome medical teams are the ones managing high-risk pregnant patients and delivering babies day and night. Your staff - from MFMs, OBGYNs and endocrinologists to certified diabetes educators, nurses, and perinatal specialists - work to ensure positive outcomes to reduce the high costs of complications. In 2013, the combined hospitalization costs of women with GDM and pre-existing diabetes was more than $1.4 billion, or about 8.5% of all hospital costs associated with pregnancy care. That number does not include NICU stays and other complications. We welcome pilots with you to prove our system works. We’re open to all kinds of collaborations. Our main goal is to help you treat patients.
How We Help Partners
Hello Potential Partners - we're thinking you might be insurers, healthcare systems, and anyone interested in solving GDM! We’re eager to meet you. We know it’s often you who bear the high costs of GDM during pregnancy plus complications, both short and long term. You know that poorly managed blood glucose levels prior to conception are more likely to result in infants with congenital birth defects occurring in the first 8 weeks of development. Autism is also related to early unmanaged GDM. The effects of genetics in the role of GDM is a fascinating area of research that will have profound effects on future generations. Pregnancy is complicated. We don’t pretend to have a silver bullet, but we strongly believe that our GlucoseMama Kits are a much-need and urgent solution.
Challenge Mission
Market Size
Werbie has an opportunity to enter into a TMO of at least 700K GDM patients: About 500,000 diagnosed with GDM during pregnancy and about 200,000 women with pre-existing diabetes (type 2) going into pregnancy every year. Our conservative estimate of 700K patients diagnosed with GDM is based the NCBI article, GDM: Risk and Management During and After Pregnancy, current statistics of up to 17% of the pregnant population depending on the type of diagnostic test used and the population studied. The occurrence of diabetes in women of childbearing age has doubled in the last 10 years, affecting 1.3 million women nationwide. According to the International Diabetes Federation, the global rate of GDM in 2015 was 212 million women. The GDM market is evergreen, renewing and growing every year as more women who are at-risk for GDM become pregnant.
Projected 3 Year Growth
In three years we intend to capture at least 10% of the market with revenues around $83M. With our clinical trial results and pilot studies, we will be in an excellent position to raise a seed round to capture 10% of the market by 2020. The international market is also on our radar.
How We Will Make Money
Werbie is in the pre-revenue stage. We aim to establish paid pilots in 2018. Our B2B business model is focused on healthcare systems, insurance companies, hospitals, Medicaid, and payers as customers. Our benefit to them is two-fold: cost savings and improved outcomes. Distribution of the GlucoseMama Kits will be through doctors and nurse practitioners prescribing the kits to their patients with pre-existing diabetes or GDM. Down the road with clinical validation, reimbursement for the kits will ideally be bundled under one code.
We are 150% dedicated to helping under-served women on Medicaid. CMS has made diabetes management a key priority. The agency is evaluating software applications that may help manage Clinical Decision Support (CDS) for Electronic Health Records. In 2011, the FDA began compiling guidelines to map the approval process for software that CMS can use for certified diabetes educators. The FDA has delayed the publication of those guidelines. We will continue to pay close attention to these developments. With IRB data we are in an excellent position to take advantage of this and to meet any regulatory hurdles to stay competitive.
About our Competition
Our biggest competition is highly ineffective pen-and-paper logging. Our unique advantage in this space is that we understand the dynamic partnership that needs to exist between the patient + medical world + technology. We thoroughly understand the disease, and the pain points of all the stakeholders. With this deep knowledge, we have developed a product that services everyone.
Progress with Customers to date
We have an on-going clinical trial with the University of Maryland Medical Center. We have a pilot with MFM Associates of Maryland in the works. We are looking for pilots with hospitals, healthcare systems, and clinics.
New Orleans and Our Company
If we win this challenge we would love to work with the wonderful people of New Orleans. The gestational diabetes rate in Louisiana is alarming and continues to rise. A report entitled, The Burden of Diabetes in Louisiana by the ADA, states that: “Approximately 521,294 people in Louisiana, or 13.9% of the adult population, have diabetes. Of these, an estimated 124,000 have diabetes but don’t know it, greatly increasing their health risk. In addition, 1,272,000 people in Louisiana, 37.5% of the adult population, have prediabetes with blood glucose levels higher than normal but not yet high enough to be diagnosed as diabetes. Every year an estimated 32,000 people in Louisiana are diagnosed with diabetes. Diabetes and prediabetes costs Louisiana $5.4 billion dollars each year.”
The pre-diabetes population, especially women of childbearing age, is the group that we are most concerned about. They are at risk for gestational diabetes and type 2 postpartum, and their children are at an increased risk for obesity and diabetes. We want to be a key player, along with other stakeholders in the healthcare system, to help these women self-manage their diabetes during pregnancy, and to offer educational tools and support for sustainable behavior change to prevent type 2. It would be an honor to officially launch the GlucoseMama Kits in New Orleans through pilots. PS: We know the food in New Orleans is totally amazing, so carb counting would be a constant challenge. But we’re up for it.
Innovation Details
Intellectual Property Summary
We have filed for a provisonal patient.
Clinical Information
Werbie was awarded a research grant from the Maryland Industrial Partnership (MIPS) for Phase 1 trials. In collaboration with the University of Maryland Medical Center MFM Department in Baltimore, we are currently engaged in an IRB-approved randomized clinical trial with 120 patients: “Improving GDM Outcomes with Prenatal Group Therapy and GlucoseMama.” Our trial aims to prove an increase in compliance with glucose tracking, a reduction in the need for medications/insulin, improved outcomes for moms and neonates, and improved compliance with post-partum visits. We will share preliminary data when it is available.
Other research in the gestational diabetes space supports the urgent need for our GlucosaMama Kits. A study in American Journal of Public Health entitled, “Percentage of Gestational Diabetes Mellitus Attributable to Overweight and Obesity” Shin Y. Kim, MPH, Lucinda England, MD, MSPH and others, concluded that: “If all overweight and obese women (BMI of 25 kg/m2 or above) had a GDM risk equal to that of normal-weight women, nearly half of GDM cases could be prevented. Public health efforts to reduce pre-pregnancy BMI by promoting physical activity and healthy eating among women of reproductive age should be intensified.” Our GlucoseMama Kits can be used with patients before conception to help improve their eating habits for weight loss and positive lifestyle changes.
Another study in the Indian Journal of Endocrinology and Metabolism entitled, Gestational diabetes mellitus: Non-insulin management by Navneet Magon and V. Seshiah reminds us that “Medical nutrition therapy is the cornerstone of therapy for women with GDM. Surveillance with daily self-monitoring of blood glucose has been found to help guide management in a much better way than blood glucose checking in labs and clinics, which tends to be less frequent.”
Our GlucoseMama Kit is focused heavily on Medical Nutrition Therapy (MNT) and blood glucose self-management. An excerpt from the Conclusion section of this study reads: “GDM is a window of opportunity for prevention of diabetes in future life. The opportunity provided by GDM can be utilized only if optimal medical and obstetric care is provided to the antenatal patient with GDM. Optimal management of GDM remains a challenge for the obstetricians and endocrinologists. MNT is the most common therapy, which suffices for GDM, but when required, pharmacological treatment becomes necessary.”
Regulatory Status
The GlucoseMama Kit in its current form does not need FDA approval.
How we will use the funds raised
Funds raised will be used to upgrade report features in the backend; enhance the nutrition platform; add elements of gamification, and produce kits. Our overall financial plan is to start a seed round in Spring 2018 once we have data from our clinical trial.
Thank You
As the rate of diabetes during pregnancy continues to skyrocket across the nation, our team at Werbie is committed to working with physicians, clinical staff, hospitals, health systems, and insurer care coordinators. Our goals are: 1) to increase patient compliance with self-management; 2) to provide care teams with the actionable data to guide treatment; and 3) to cut costs while improving outcomes. Our in-house medical team - including OBGYNs, an FNP-OB specialist, perinatal RD/LD, RDN, and CDE - works in tandem with our software developers to keep patients and doctors happy. Pregnancy is a special time in a woman’s life. Our goal is to make every mom and baby as healthy and happy as possible. We are looking for pilot studies to prove our system works. Please reach out if we can serve you.
Updates
No updates found .
Supporters
-

01/20/2018 - Liked the project.
01/19/2018 - Liked the project. , MS Information Systems Management
, MS Information Systems Management
01/18/2018 - Followed the project. , MS Information Systems Management
, MS Information Systems Management
01/18/2018 - Liked the project.
01/18/2018 - Liked the project.
01/17/2018 - Backed the project for $10Instant Feedback
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.
59Medstartr
Index Score59
Interest
Score0
Adoption
Score6
Likes0
Partners0
Pilots2
Follows-
This campaign has ended but you can still get involved.See options below.
$10 pledged of $10,000 goal
Instant Feedback
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.


