MED GARB: NORMOTHERMIC GARMENT SYSTEM
Hypothermia with surgery is more than just being cold. It leads to a cascade of surgical complications from wound infections to heart attacks. This cozy garment prevents hypothermia and looks sharp.
NORWALK, CT United States Wearables Medical Device Equity RaiseAbout our project
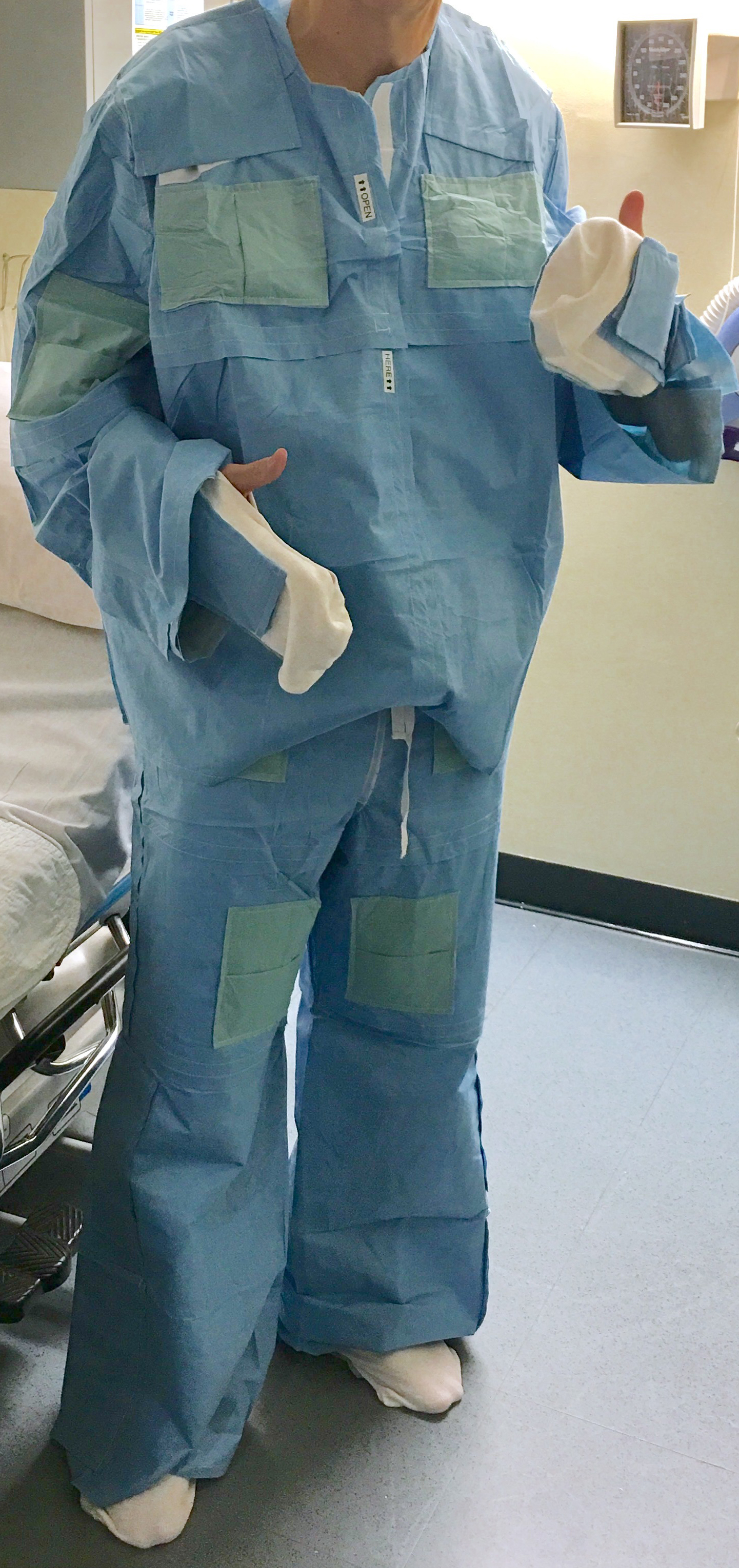
The problem we solve: Unintended Hypothermia associated with surgery can be a deadly and an expensive health care event which increases surgical complications such as wound infection, blood clots, bleeding, cardiac arrhythmias and heart attacks. Unintended Perioperative Hypothermia (UPH) is an epidemic which prolongs hospital stay by 20% increasing health care costs in the billions of dollars. Most warming devices intended to correct UPH, are tethered to a power source, are cumbersome, noisy and prevent the patient moving freely before and after surgery. The SNUGS study garment is silent and does not radiate heat into the environment. It is low-profile, self-heating, eco-friendly, comfortable and it approximates normal clothing such as a pair of pajamas with socks. The patient applies the garment without assistance after undressing on admission, before surgery
About our solution: The device is like normal clothing. It comes in a flat vacuum pack which the patient opens. The patient can apply the jacket, pants and gloves with or without assistance. The chemical heat packs in the garment are activated on exposure to air, when the pack is opened. Optimal temperature is reached in 20 minutes and last for 12 hours. Some of the advantages over the competition, which either use plastic inflatable bubble blankets connected to a hot-air blower or some kind of re-usable electric powered plasticized sheets, are that the garment is silent, single-use- ecofriendly, low-profile, comfortable and allows free unencumbered movement. Technology is simple, and all of the components of the device are in use in the medical field. The device is light and compact and portable. It can be used in a wide variety of medical scenarios such as search and rescue, military and third world. Lastly it is intended that the device will replace the ubiquitous cotton ‘Johnny-Coat’ used in Hospitals
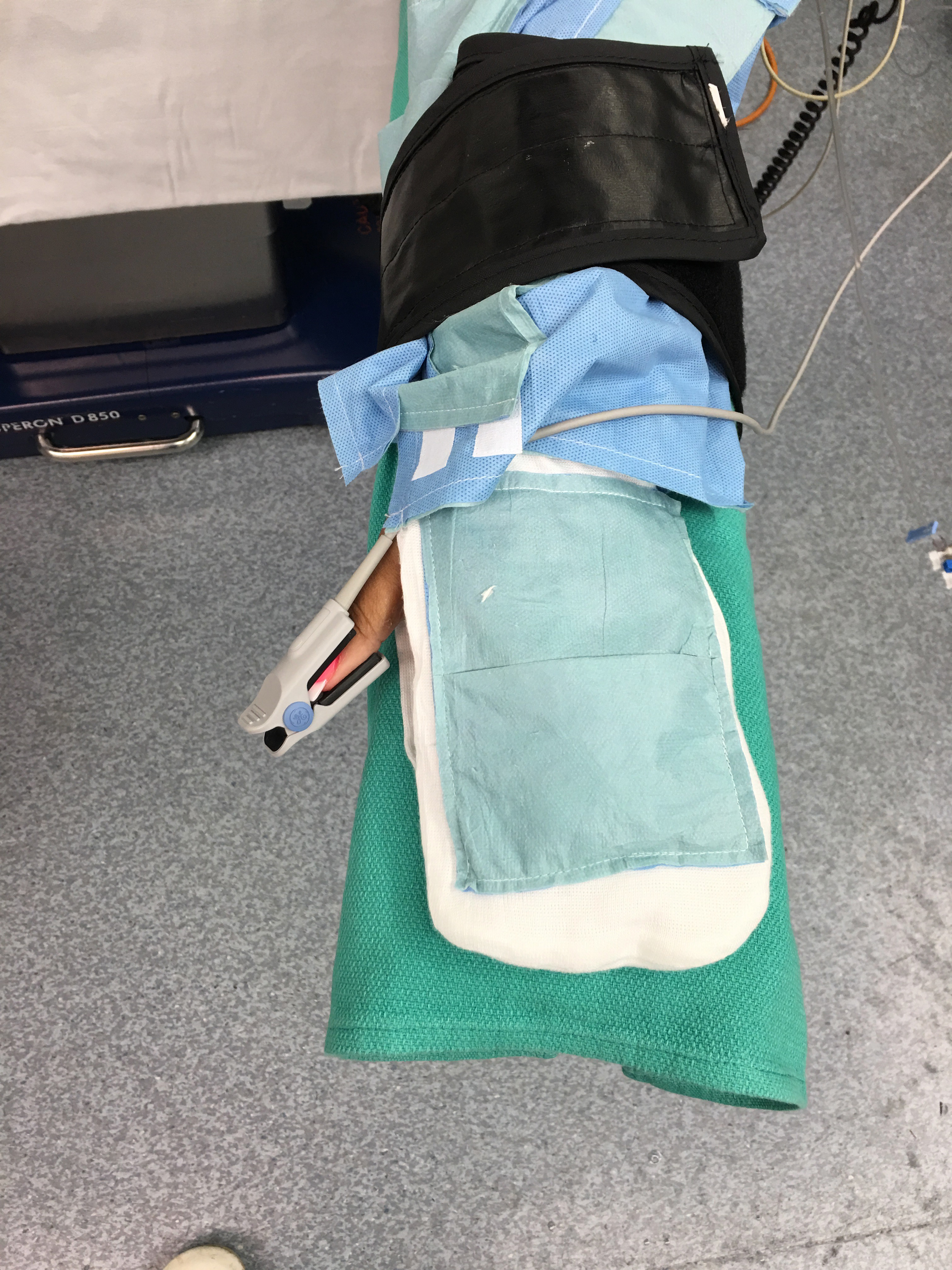
Progress to date:
IRB Clinical Trial Completed and published.
Comparison of Patient Warming System with Integrated Air-Activated Heat Packs versus Forced-Air Warming Device. Randomized Controlled Clinical Trial to Evaluate Feasibility and Safety. Int J Recent Sci Res 9(5), pp. 26762-26768, May 2018.
The study was registered with the National Institute of Health ClinicalTrials.gov PRS: NCT02905708, with the approval of the Norwalk Hospital Institutional Review Board and with prior written informed consent, we studied 48 participants undergoing elective surgery. This study was designed primarily as a ‘proof of concept’ study to demonstrate that the integrated heatpack garment was effective in maintaining normothermia during the perioperative period.
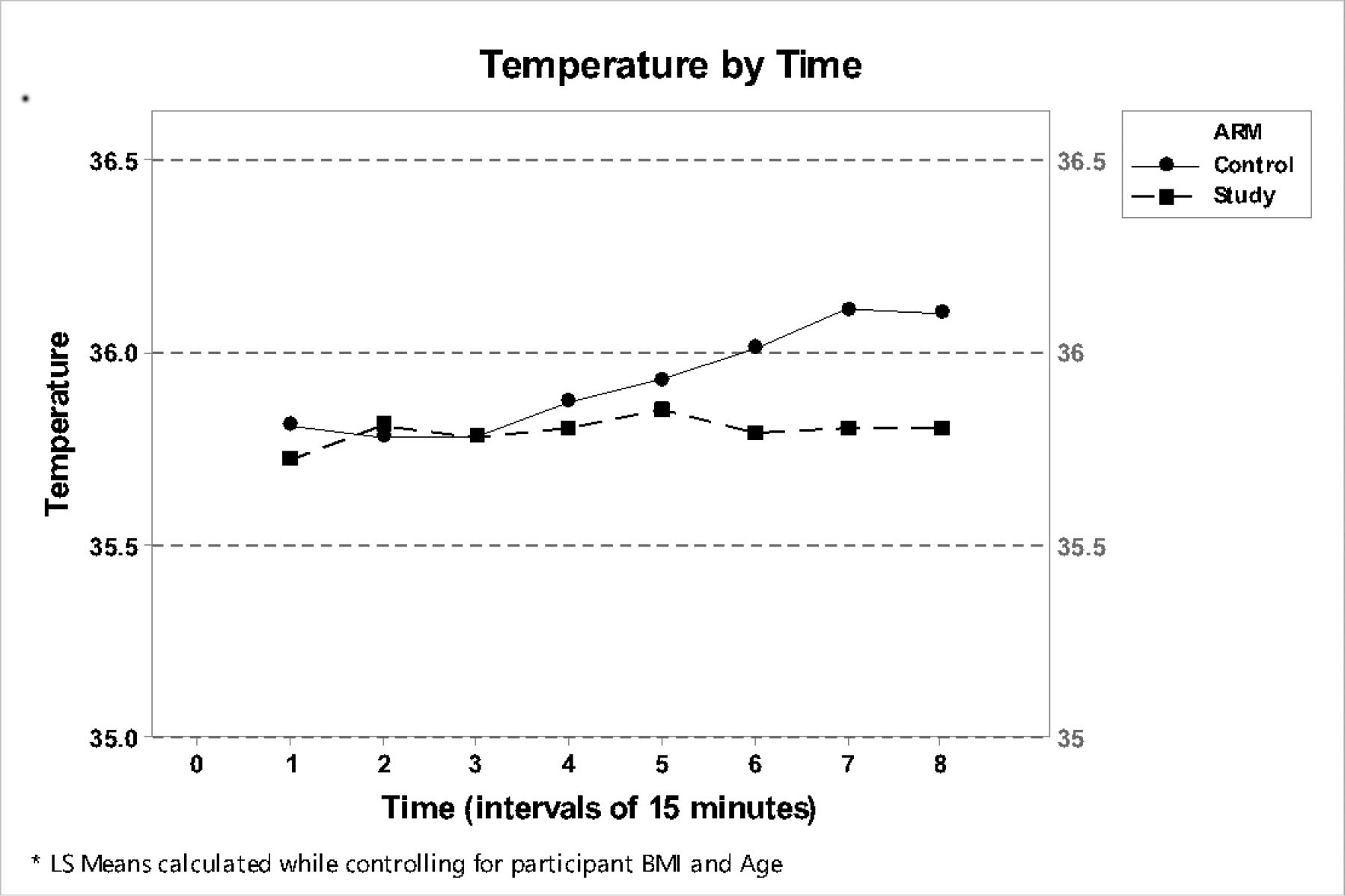
There was no statistical significance between the two groups for the first 120 minutes of anesthesia. Thereafter the Control group had significantly higher temperatures although the number of participants in the Control group was higher. All of the patients in both groups were normothermic on admission to the PACU thereby conforming with the NICE guidelines. No patient felt cold or experienced clinical or ECG manifestations of shivering in the PACU. This initial study with a prototype garment, demonstrated that the pajama like garment with gloves and socks and integrated heat packs, is not only more user friendly but also an effective means of patient warming in the perioperative period. The garment had other demonstrable advantages over the resistive polymer (RP) and Forced Air Warming Devices (FAWD).
1. It is a single use device unlike the RP or the FAWD device.
2. It is ‘ecofriendly” All of the components are biodegradable.
3. It requires no external power supply, controller or device.
4. There is no umbilical to immobilize the patient. The device not ‘tether’ the patient, keeping them immobile.
5. During ambulation to the bathroom in the admissions area and during transfer to the OR (ambulatory or on a stretcher) the patient does continues to get the benefit of the warming garment.
6. The garment simulates normal clothing and is self-contained; which adds the In-built advantages of requiring:
a. little or no input form the care givers.
b. makes the patient feel warm, secure and comfortable in an alien, distressing environment.
The provision of a garment that approximates normal clothing adds to patient satisfaction levels. The little as well as the ‘big’ details improve patient care delivery and reduces deleterious results in any High Risk Organization. In summary, this randomized study prospective study indicates that intraoperative core temperatures were the same in both study groups, for the first 120 minutes of general anesthesia and both groups were normothermic on admission to the Post Anesthesia Recovery Unit (PACU). A pajamalike garment with integrated heat packs is not only user-friendly but has been shown to be an effective means of patient warming in the perioperative period.
Prototype was used in the IRB Study. The Patients, Nurses, Surgeons and Anesthesiologists all liked it. One patient liked it so much, he kept it on for several days after he was admitted to the ward and the nurses wanted to replace it with a standard 'Johnny-Coat'.
About Our Team

Creator: Laurence Kirwan
Location: Connecticut
Education: UNIVERSITY OF MANCHESTER, ENGLAND
Bio: Laurence A. Kirwan MD, FRCS, FACS (K) 30+ years of Aesthetic Plastic Surgery MB ChB Victoria University of Manchester, 1974 Fellow of the Royal College of Surgeons England,1979 Diplomate American Board Plastic Surgery, 1989 Certificate of Added Qualifications Surgery of the Hand, 1990 Fellow American College of Surgeons,1992 Responsible for intellectual property creation and design
Hospital Affiliation: NORWALK HOSPITAL, CT, MT. SINAI HEALTH SYSTEM, NEW YORK.
Title: Director CSO
Advanced Degree(s): MD, FRCS (ENG), FACS, DIPLOMATE AMERICAN BOARD OF PLASTIC SURGERY
About Team Members
Stephen McLoughlin
Director, Business Operations, B.A., Dipl. B.A., FGS, SPE
Biography: University of Warwick - Warwick Business School
Keele University – Faculty of Law
Geo-Steering Engineer at Geo-Steering LLC
Senior Directional Driller at Schlumberger Drilling & Measurements
President, Rotary Steerable Tools Inc.
Responsible for design, writing patents, legal documentation
Title: Director, Business Operations
Advanced Degree(s): B.A., Dipl. B.A., FGS, SPE
Justine Taddeo
Director, Product Design, NA
Biography: 24 years in the Garment Design Industry for the UK & USA markets
Responsible for research & analysis of fabric, & the physical & material design of the gown
Title: Director, Product Design
Advanced Degree(s): NA
About Our Company

KMT TECHNOLOGIES LTD
Location: 148 East Avenue
Suite 2A
NORWALK, CT 06851
US
Founded: 2016
Product Stage: Prototype/MVP
YTD Sales: Working on it
Employees: 1-2
How We Help Patients
Less complications associated with surgery means a longer healthier life. ‘It all starts with patients’. As a Plastic Surgeon for over 30 years and as an inpatient and an outpatient on several occasions, I have been personally struck with the inadequacies of the ubiquitous ‘Johnny-Coat’ or hospital gown. For those of you who don’t know what this is, it is a thin cotton or paper, smock-like garment which opens and closes inconveniently, with ties, in the back!
The ‘MedGarb Solution’ promotes patient comfort and warmth. The heat source is completely integrated and does not require any external power source or device. It is as easy to apply as your own clothes. Heat is safely generated by integrated iron-oxide, non-toxic heat packs which are activated when the package is opened. The MedGarb consists of a pair of pants, a jacket which closes in the front and an optional pair of gloves, with the thumbs cut off. The patient puts the garment on when they are admitted and takes them off on discharge. The garment is secure but loose fitting and non-restrictive. Moreover, the MedGarb preserves a sense of patient dignity and patient modesty.
How We Help Physicians
What’s good for the patient is good for the provider and the hospital.
At the very basic level, a great experience and a high satisfaction level translates into a happy patient, great word-of-mouth referral, and a patient who returns to that provider or hospital.
When you put a patient in a Johnny-Coat you get the opposite experience. You create insecurity, embarrassment, stress and hypothermia. I know, I’ve worn a ‘Johnny-Coat’ on numerous occasions. If you sleep in a Johnny Coat, it tightens and twists and the knots on the back make it uncomfortable. I know, I’ve slept in one. When the Physician tells you to ambulate, you stroll down the corridor holding your iv stand in one hand and the back of your gown with the other so that your butt isn’t hanging out. I know, I’ve done it.
How is it that we as physicians have such a blinkered tunnel view as to not appreciate the inadequacies of the standard hospital gown? As a paper or a cotton version, it has little to no heat retention capability and even if it did, the short sleeves, smock design ending at the knees and last but not least the full length opening at the back, make any body-heat retention a mathematical and scientific impossibility.
Johnny Coats serve no useful function. They promote Hypothermia in a hospital or out-patient settings and may act as carriers of infection even after laundering. They fail to cover the patient in an adequate fashion. They fail to keep the patient warm or comfortable and they impair the patient’s ability to mobilize outside of their hospital room because of the inadequate coverage.
The dedicated devices They cannot go to the bathroom without assistance, cannot even stand up. Their source of heat is interpreted as soon as they leave the admissions area and are moved to and from the operating room.
How We Help Hospitals
Complications arising from Unintended Perioperative Hypothermia increase hospital stay by 20% with significant unanticipated costs to the provider. Furthermore, these additional costs may not be covered by insurance if due to UPH. https://qpp.cms.gov/docs/QPP_quality.../2017_Measure_424_Registry.pdf
The most common methods in use to prevent UPH are Forced Air Warming Devices (FAWD) and Resistive Polymer blankets (RP). Each is connected to a power source. Additional equipment, assistance and monitoring is required. FAWD’s are noisy, bulky and radiate heat. Ambulation to the bathroom, before or after surgery, requires discontinuation of the active warming process. Transfers to and from the operating room require termination of active warming. Cotton blankets cotton hospital gowns are used to supplement these devices. This adds additional costs. Neither FAWD or RP devices are transferable to the standard in-patient setting.
In contrast, the MedGarb solution is a universal garment which replaces the FAWD, RP and the hospital gown. It is constructed of two-layers of proprietary insulated materials which are intended to insulate whilst allowing evaporation of perspiration. It is in the form of a jacket, pants or shorts and gloves and is combined with standard issue non-slip socks. The garment is intuitive and requires little or no assistance and no provider monitoring. There is no tether and no power input. Warming is continuous and uninterrupted during mobilization and transfers. The garment is silent, low profile and requires no additional cotton blankets or hospital gowns to enable its use. Heating elements which are integrated in the garment, consisting of air-activated non-toxic heat packs effective for up to 12 hours.
The patient remains in the garment until discharge. With or without heat packs, this is a ‘Universal’ garment for patient wear. Applicable to both sexes and all ages. Disposable, sterile and eco-friendly. A one stop solution for preventing UPH in a cost-effective package.
How We Help Partners
Forced Air Warming Devices are the most commonly used patient warming devices. CMS (Centers for Medicare and Medicaid Services) recommends them. However, the market leader (Bair-Hugger manufactured by 3M) has several drawbacks. First and foremost, it is bulky and requires an additional machine in an already crowded operating room environment with an additional electrical cord. The device is noisy and contributes heat by conduction and radiation to the surgeon and assistants. It requires a blanket and a strap to hold in place and is only functional when connected to a power supply so that patient receives no warming during transfer back and forth to the operating room and when ambulating to the bathroom. Worst of all is that there are numerous studies demonstrating that the hot air exiting from the device drives dust and bacteria from the floor upwards into the surgical wound causing a catastrophic increase in surgical site infections (especially joint surgery) in which one germ can cause an infection. The result of these infections is an increase in morbidity and mortality with increased length of hospital stay and exponential increases in hospital costs. The MedGarb system is a simple, technologically elegant, solution to patient warming which eliminates all the deficiencies of the Forced Air technology. It functions throughout the perioperative experience without interruption and requires no additional machinery, electrical supply or provider input. As the preferred provider of conductive heat technology, it is anticipated that MedGarb will be the market leader displacing Forced Air devices in a market worth more than one billion dollars per annum in the US and Europe alone. In addition, because of its portability and simplicity, the device has applications in out-patient and non-surgical procedure settings significantly increasing its potential market reach four to five-fold to 300 million patient interactions per annum including an estimated 60 million surgical procedures.
Innovation Details
Intellectual Property Summary
Intellectual Property Status Patent Applications Filed
The device is the subject of 3 patent applications (see links below):
1. Normothermic Maintenance System & Method Filed 29th May, 2013 Class 601/18, International Class A61F USPTO Application Number 14/890280 / 13986724
2. Normothermic Maintenance Method & System Filed 5th October, 2013
Published PCT/EP 2014/059528 EP 2994081 5th March 2016 EPO Application Number 14 7277214
3. Garment Apparatus, System & Method Filed 5th October, 2013
PCT EP 2014/059534 US 61855221
The collective purpose of these patents is to provide protection of the invention of a stand-alone garment with self-contained heating system with particular reference to targeting the application to heat zones which are located on the periphery of the patient. The objective of this design – which has since proved correct in clinical trials – was to prevent heat re-distribution from the patient's core. The principle on which it works is that, if the temperature of the patient’s extremities is maintained then there is no physiological driver for heat to be diverted from the patient's core to the periphery. This, in turn, reduces the effect of surgically induced hypothermia, reducing recovery time and improving the patient's chances of of an uneventful recovery.
Intellectual Property status:
Med I
Under examination. Introduces the concept of an "arms and legs only" coverage heating system.
Med II
Under examination – probably the least valuable of the 3 patents, but covers the niche of clothing with enclosed heating means being deployed only to the lower legs, feet, forearms and hands.
Med III
Under examination – reveals a segmentable clinical whole body coverage garment with re-attachable seams, enabling the patient to be operated on while continuing to provide passive insulative heating to the core and active heating to the periphery. Works in conjunction with Med I to provide full coverage.
Clinical Information
IRB Clinical Trial Completed and published.
DOI: http://dx.doi.org/10.24327/ijrsr.2018.0905.2117
Comparison of Patient Warming System with Integrated Air-Activated Heat Packs versus Forced-Air Warming Device. Randomized Controlled Clinical Trial to Evaluate Feasibility and Safety. Int J Recent Sci Res 9(5), pp. 26762-26768, May 2018.
The study was registered with the National Institute of Health ClinicalTrials.gov PRS: NCT02905708, with the approval of the Norwalk Hospital Institutional Review Board and with prior written informed consent, we studied 48 participants undergoing elective surgery. This study was designed primarily as a ‘proof of concept’ study to demonstrate that the integrated heat-pack garment was effective in maintaining normothermia during the perioperative period.
There was no statistical significance between the two groups for the first 120 minutes of anesthesia. Thereafter the Control group had marginally higher temperatures (maximum temperature difference was <0.5°C) although the number of participants in the Control group was higher. All of the patients in both groups were normothermic on admission to the PACU thereby conforming with the NICE guidelines. No patient felt cold or experienced clinical or ECG manifestations of shivering in the PACU. Several patients commented positively on the feel and fit of the garment. This initial study with a prototype garment, demonstrated that the pajama like garment with gloves and socks and integrated heat packs, is not only more user friendly but also an effective means of patient warming in the perioperative period. The garment had other demonstrable advantages over the resistive polymer (RP) and Forced Air Warming Devices (FAWD).
- It is a single use device unlike the RP or the FAWD device.
- No potential for MRSA transmission
- It is ‘ecofriendly” All of the components are biodegradable.
- It requires no external power supply, controller or device.
- There is no umbilical to immobilize the patient. The device not ‘tether’ the patient, keeping them immobile.
- During ambulation to the bathroom in the admissions area and during transfer to the OR (ambulatory or on a stretcher) the patient does continues to get the benefit of the warming garment.
- The garment simulates normal clothing and is self-contained; which adds the In-built advantages of requiring:
a. little or no input form the care givers.
b. makes the patient feel warm, secure and comfortable in an alien, distressing environment.
The provision of a garment that approximates normal clothing significantly adds to patient satisfaction levels. The little as well as the ‘big’ details contribute to improving patient care delivery and reduces deleterious results in any High Risk Organization.
In summary, this randomized study prospective study indicates that intraoperative core temperatures were the same in both study groups, for the first 120 minutes of general anesthesia and both groups were normothermic on admission to the Post Anesthesia Recovery Unit (PACU). A pajama like garment with integrated heat packs is not only user-friendly but has been shown to be an effective means of patient warming in the perioperative period.
Regulatory Status
IRB Clinical Trial Completed and published. The next step is finalization of pattent applications and / or application for 510(k) Certification as a Class II device based on a predicate device (Barrier EasyWarm Blanket, Bair-Hugger Forced Air Warming Device or similar patient warming device). Similarly application for a CE Mark in the EU. See Use of Funds below.
How we will use the funds raised
Completion of Patent Applications through to final examination stage and award in US and EU. Estimated cost $35,000.00
FDA 510(k) Clearance Product Code: DWJ, Class 2 Device. $35,000.00 This cost could be reduced if the partners make their own application, reducing the requirement for professional assistance. This number was included as insurance.
CE Mark $15,000.00 Can probably use some of the materials for the FDA 510 (k) clearance.
Registration of Trademark. $15,000.00. This cost can be deferred until required.
Thank You
As a Surgeon for over 30 years I am well acquainted with the dangers of Hypothermia with surgery. It is a MAJOR health risk and prolongs hospital stay form the average of 4 days to 5 days. Preventative methods include hospital gowns, cotton blankets, and Forced Air (FA) devices. FA devices are poorly fitting and are tethered to a power source. FA devices increase noise, heat and wound site infections during surgery. Warming is discontinued to allow the patient to use the bathroom and during transfer to and from the operating room. MedGarb is a pajama like garment. It is easily applied and has integrated air-activated heat packs. There is no power source or tether. MedGarb is adaptable to a wide variety of settings in and out of the hospital. MedGarb is low tech, disposable and eco-friendly.
Investor Info
Market Size
The Total Market Opportunity (TMO) represented by the perioperative surgical hypothermia market is defined by how many surgeries are performed. 48 million surgical inpatient procedures were performed in the United States in 2009, 58 million per annum if you include the EU. The minimum use for one heating device per surgical event. The estimated annual use in the EU and US combined is therefore 58 million Assuming a unit sale price of $25.00 the estimated potential gross market sales are $145,000,000 per annum. It is anticipated that market share will be available in Canada, Australia, Asia, and South America.
Assuming a unit sale price of $25.00 this results in a total market opportunity for our products of $145,000,000 per annum in the US and UK alone. 3M purchased the Forced Air Warming Device (Arizant) Technology in 2010 for $810 million.
September 9, 2010 7:30 am CDT
ST. PAUL, Minn.--(BUSINESS WIRE)--3M announced today it has entered into a definitive agreement to acquire Arizant Inc. for a purchase price of $810 million in cash. Based in Eden Prairie, Minn., Arizant is a leading manufacturer of patient warming solutions designed to prevent hypothermia in surgical settings.
“Patient warming is a highly strategic adjacency for our business and integral to infection prevention,” said Debra Rectenwald, president and general manager, 3M Infection Prevention Division. “In addition to Arizant’s market-leading products, its rich intellectual property portfolio and solid technology platforms expand 3M’s infection prevention offerings and will help to drive growth internationally, as well as provide a platform of innovative wound care technologies.”
Arizant created the category of forced-air patient warming with the introduction of Bair Hugger® therapy in 1987. The global opportunity for patient warming is approximately $1 billion, with the forced-air warming category expected to grow at about 10 percent per year.
Projected 3 Year Growth
We intend to achieve a conservative 1% market penetration across the North American and European markets. Assuming a unit sale price of $30.00 per garment, this equates to 580,000 garments and gross profits of $17.4m by 2021. After achieving and confirming marketability and increasing market presence, increasing growth potential with more aggressive expansion will be possible through franchising the license for more remote locations, (Middle East, Africa and Asia Pacific) and will allow us to expand our platform to include products and services that can be used within the generalized hospital setting as well as outside the hospital.
- In 2014, for example, there were 33.1 million hospital admissions at community hospitals in the United States, and each patient stay would last for an average of 5.5 days accounting for a total in-patient day stays of 182 million. In 2011 the number of outpatient department visits was 125.7 million. Source: National Hospital Ambulatory Medical Care Survey: 2011 Outpatient Department Summary Tables, table 1[PDF – 330 KB]. There is therefore an additional 307 million potential patient events in which a MedGarb could be applied (see below).
Revenue Model
Our device is extremely useful for the four main stakeholders of healthcare innovation (Patients, Providers, Partners, and Institutions) with benefits at all levels. Patients benefit with greater comfort, improved outcomes and lower rates of complications. Providers benefit with an improved patient experience, ease of use and lack of noise and heat pollution in the surgical environment. Less assistance and monitoring is required with savings in time and labor costs. The device is disposable and eco-friendly. Partners and institutions benefit with greater patient and provider satisfaction and reduced cost in terms of a eliminating reusable cotton gowns and blankets, reduced complications and reduced hospital stays. Income is based on unit sales. More sales, more income. Units are sold on an institutional rather than an individual basis.
- In 2014, for example, there were 33.1 million hospital admissions at community hospitals in the United States, and each patient stay would last for an average of 5.5 days accounting for a total in-patient day stays of 182 million. In 2011 the number of outpatient department visits was 125.7 million. Source: National Hospital Ambulatory Medical Care Survey: 2011 Outpatient Department Summary Tables, table 1[PDF – 330 KB]. There is therefore an additional 307 million potential patient events in which a MedGarb could be applied (see below).
als
Competitors
KMT is unique. Factors in our favor are the proprietary design and materials, patent applications and a completed IRB study. We believe that our approach to maintenance of Normothermia and patient comfort is a new paradigm and a market disruptor. Moreover, the current market leader-Bair Hugger by 3M- is a forced air warming device (FAWD) which has several inherent drawbacks. These are its reliance on tether to a power source and a hot-air blower as well as the environmental noise and heat pollution generated by the unit. The patient is immobilized and the units is bulky and restrictive. It requires constant attention and monitoring by the medical providers. The FAWD is plastic and non-ecofriendly. In addition, there are numerous studies documenting an increased infection rate with the FAWD and several health organizations in the US and the UK have already discarded forced air warming in favor of conductive heating in order to reduce their infections rates in orthopedic cases and generally. The initial IRB trial units were received favorably by all levels of the staff from nurses in the admissions area to surgeons and anesthesiologists. They keep asking ‘when is it coming out?’
Traction
- Making the market is going to be by traditional medical sales techniques of offering free samples. However, rather than adopting an initial aggressive displacement marketing stance, an alternative, supporting role suggests itself. In this, high risk category patients may use the FAWD device or the KMT garment. It is also anticipated that the MedGarb will be an intuitive sell for a whole array of ambulatory non-surgical procedures such as upper and lower GI Endoscopy, Cystoscopy, D&C, cervical biopsy, skin lesion excision, bronchoscopy, cataract surgery as well as minor head and neck surgery such as tonsils and adenoids, ear tubes, otoplasty, nose surgery, eyelid surgery and face-lifts. It is especially well suited to upper and lower extremity surgery such as shoulder, elbow, wrist, hip, knee, ankle and foot. The MedGarb is also an ideal adjunct for all ambulatory investigational procedures such as CAT scans, MRI’s, Ultrasound exams, Mammography, Cardiac Stress Tests and EMG’s. The ubiquitous hospital gown can also be replaced with MedGarb with or without heating packs. All of these additional applications open the potential use to a market much greater than surgical procedures alone. In 2014, for example, there were 33.1 million hospital admissions at community hospitals in the United States, and each patient stay would last for an average of 5.5 days accounting for a total in-patient day stays of 182 million. In 2011 the number of outpatient department visits was 125.7 million. Source: National Hospital Ambulatory Medical Care Survey: 2011 Outpatient Department Summary Tables, table 1[PDF – 330 KB]. There are therefore an additional 307 million potential patient events in which a MedGarb could be applied. This will allow the medical staff greater flexibility in their approach while guaranteeing normothermia in the patient.
Due Diligence Docs
Please note that access to the company's confidential materials is limited. Click this button to request access from the Company and its representatives.
Updates
No updates found .
Supporters
There are not supporters yet.
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.
0
Score
0
Score
0
Likes0
Partners0
Pilots0
Follows-
This campaign has ended but you can still get involved.See options below.
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.


