Nottingham: Illusion Therapy Smartphone App
Augmented reality app that "virtually" stretches and compresses fingers, which, as part of a healthy lifestyle, may help individuals live well with joint pain associated with arthritis.
Encinitas, CA United States Healthy Living Pain Management Alternative Therapies Affordable Choices challenge Access to Care challenge NOLAHI Challenge - See AllAbout our project

The problem we solve: The leading cause of disability among adults is arthritis, an informal way to describe joint pain or joint disease. There are 54 million Americans have arthritis. That is 1 in 5 adults and even 300,000 children. 50% or 27 million are 65 and older. It is estimated to grow to 78 million arthritis sufferers by 2040. Medications commonly prescribed for the pain associated with arthritis produce side effects that render them intolerable or too dangerous for many patients.

About our solution: Nottingham is a smartphone app. It is an accessible, drug-free, digital alternative treatment. It uses the scientific principles of Illusion Therapy (IT) to cause the brain to "see" the therapeutic application of stretching and compressing the pained joints. Illusion Therapy is the process of manipulating the appearance of painful body parts in order to reduce pain. University of Nottingham UK study of 20 osteoarthritis sufferers with diagnosed arthritis in hands used IT to virtually shrink and stretch the painful hands and fingers. The therapy was effective. 85% reported level of pain was reduced by 50%. Phantom Limb Mirror Therapy is a form of IT and studies have shown that illusions or imagery of movement of the amputated limb alleviates phantom limb pain. The problem with IT for arthritis is accessibility and scalability. The current option is the MIRAGE box used in the UK study. It is large, expensive, and unavailable for everyday use.
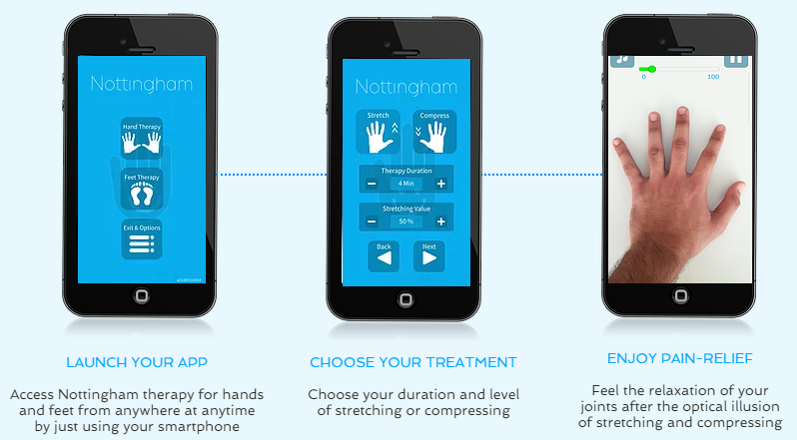
Progress to date:
A working app was approved for release in January 2019 in the Apple and Google Play app stores. Nottingham US has been beta-tested at Arthritis events in November and December 2018 with very positive response. We are currently seeking to do a pilot study through programs such as this in order to classify Nottingham as an evidence-based treatment. The goal of the study would be to understand and improve the effectiveness of the Nottingham app to reduce pain associated with chronic hand and foot joint pain.
About Our Team

Creator: Paul Lafferty
Location: California
Bio: Paul has over 30 years of experience in software development and technology management experience. Paul is responsible for operations and business development for VRx Medical in the United States. Paul currently sits on the San Diego board for the Arthritis Foundation.
Title: GM
About Team Members
Garland Wong
Founder, BS Biotech UCSD
Biography: Garland Wong is a global serial entrepreneur who has co-founded numerous companies in every major continent and has been involved in raising over $50 million in venture capital.
Title: Founder
Advanced Degree(s): BS Biotech UCSD
Carter Jones
Medical Advisor, MD PhD
Biography: Medical Director, Colorado Clinic. R. Carter W. Jones III, MD PhD is board certified in both Anesthesiology and Pain Medicine and Ph.D. in Pharmacology. He has been a practicing anesthesiologist and pain management physician since 2011.
Title: Medical Advisor
Advanced Degree(s): MD PhD
Huan Giap
Medical Advisor, MD PhD
Biography: Radiation Oncologist. Director of Proton Cancer Treatment Center. CEO and Founder of Virtual Reality Medical Applications, Inc. Partner with VRx Medical on phantom limb pain VR therapy. Co-editor-in-chief of the Translational Cancer Research Journal.
Title: Medical Advisor
Advanced Degree(s): MD PhD
Christopher Rogers
Medical Advisor, MD RMSK
Biography: Dr. Christopher J. Rogers is a board certified physician in both Physical Medicine & Rehabilitation (PM&R) and Regenerative Medicine. With more than 20 years of clinical experience, Dr. Rogers is one of the world’s leading experts in the field of orthopedic regenerative medicine, specializing in the non-surgical treatment of spine and joint conditions. He has developed new approaches for the treatment of tendon injuries, osteoarthritis and disc degeneration which provide safe and viable alternatives to surgery.
Title: Medical Advisor
Advanced Degree(s): MD RMSK
About Our Company
Location: 1345 Encinitas Blvd
335
Encinitas, California 92024
US
Founded: 2018
Website: https://www.vrxmedical.com/
Product Stage: Ready
YTD Sales: Working on it
Employees: 1-2
How We Help Patients
Nottingham is a wellness product. Pain management, pain reduction, increased range of motion may help sufferers live well with these painful diseases.How We Help Physicians
Nottingham could be a low risk, low cost, accessible, complementary, alternative therapy to what care providers are already prescribing.How We Help Hospitals
Conventional treatments for osteoarthritis include medications, physical therapy, cell based therapy injections and surgery. Each of these treatment options have well known risks and benefits. Many times, the treatment will not have a lasting benefit. When these therapies fail, patients often look to sources outside conventional medicine for relief. Unfortunately, unregulated and untested treatments may carry risks unknown to the patient. Nottingham is a smartphone app that is safe and easy to use. It is based on the premise that our brain senses pain using mechanisms that are influenced by visual and other perceptions. Through this program we hope to make Nottingham and evidence-based, FDA approved therapy that hospitals can recommend as a complementary, alternative, low risk therapy.How We Help Partners
An area we are seeking partnerships is where we would have access to help the aged care population that is suffering from arthritis. This might include regional and national home care and assisted living facilities, and healthcare insurance providers. Potential benefits include incremental savings due to lower medication needs, and similar reduction in costs due to patients living better with pain.Challenge Mission
How We Address the Mission of The Challenge(s)
Access to Care Challenge The Nottingham smartphone app is a digital therapeutics solution. It is an novel, easy to use, accessible technology that can help partner care providers improve their therapy offerings.
Affordable Choices ChallengeThe Nottingham smartphone app provides access for the consumer to a low cost, low risk, accessible, complementary, alternative, self-help therapy. Regular use of the app could incrementally reduce patient reliance on conventional treatments for osteoarthritis: medications, physical therapy, cell based therapy injections and surgery. This reduction in reliance on hospital services leads to a reduction in costs and risks associated with those services. Nottingham is safe and easy to use. It is based on the premise that our brain senses pain using mechanisms that are influenced by visual and other perceptions. Through this program we hope to make Nottingham an evidence-based, FDA approved, digital therapy that hospitals can recommend.
New Orleans and Our Company
We would hire locally to manage the pilot study and meet with local resources and advisers.Innovation Details
Intellectual Property Summary
Our innovative application of Illusion Therapy brings a moving box-sized, mirror-based technology down to a smartphone app.The IP is the custom augmented reality programming needed to translate the therapy to a smartphone device.
Clinical Information
The concept for our innovation came from a study done at the University of Nottingham UK. Using a lab tool called the Mirage (https://miragelab.co.uk/about/), 20 adults with average age 70, and clinically diagnosed with hand and finger joint pain due to osteoarthritis participated in the approved study. The illusion therapy proved effective with 85% reporting the pain being reduced by 50%. https://www.researchgate.net/publication/50907150_Analgesic_effects_of_multisensory_illusions_in_osteoarthritis
Video demonstrating the illusion: https://youtu.be/7C8EYMJjfiw
We are seeking a pilot study/clinical trial for our product and assistance in getting FDA clearance and breakthrough device designation. Until then we are working under the guidance of the FDA's descriptions of wellness products.
Regulatory Status
We are seeking a pilot study/clinical trial for our product and assistance in getting FDA clearance and breakthrough device designation. Until then we are working under the guidance of the FDA's descriptions of wellness products.
How we will use the funds raised
All monies would be designated to costs associated with development, execution, results analysis of pilot study, and costs associated with getting FDA clearance.
Thank You
As one of our medical advisors, Dr. Christopher Rogers of the San Diego Orthobiologics Medical Group, put it so well: "The Nottingham software application has great potential to improve the lives of people suffering from osteoarthritis. It is affordable, easy to use, and has none of the risks associated with many other forms of therapy. With time, we will learn which medical conditions best respond to this novel therapy and influence the lives of millions of people." There are 31 million Americans suffering from the joint pain associated with osteoarthritis. Our mission is to help those people. All they need is a smartphone.
Updates
No updates found .
Supporters
-

Comments
-
G K posted on 1st July, 2020
I think this could work well as an app, as a component of pain management. I’m curious about psychiatric applications as well e.g. as a component of behaviour therapy for body dysmorphic symptoms, eating disorders etc. I realize the technique needs more evidence based research but an app could be a simple convenient inexpensive way to study this, with almost no risks involved. At worst it could be distracting, meditative, or entertaining. Is the trial of the app available in Canada?
Click here to Login -
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.
Index Score
27
Score
0
Score
2
Likes0
Partners0
Pilots0
Follows-
This campaign has ended but you can still get involved.See options below.
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.



