Engineered Cornea: Engineered Cornea
We are creating an engineered corneal implant to expand global access to corneal replacement and repair, reducing dependence upon donated corneal tissues.
Lansdale, PA United States Medical Device Regenerative MedicineAbout our project
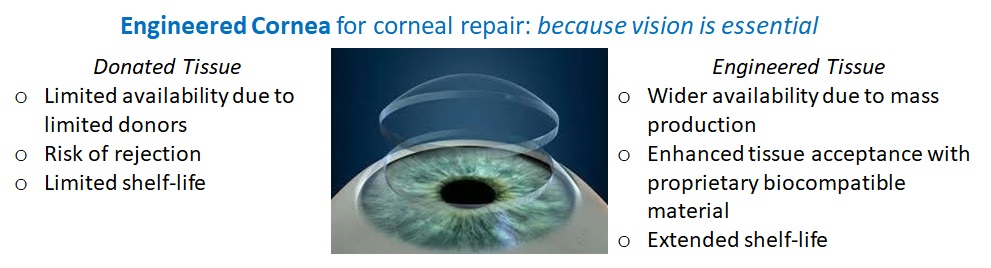
The problem we solve: We seeks to expand access to transplant materials for cornea replacement and repair and reduce the associated cost. Insufficient donor cornea tissue limits access to keratoplasty in some regions. In an epidemiological study (conducted from August 2012 to August 2013), 184,576 corneal transplants were reported by 116 countries (Gain et al., 2016). However, the total number of patients who could potentially benefit from corneal transplantation may far outnumber the few who receive the surgery. Per a 2010 global estimate, corneal opacities account for 1% of visual impairments (inclusive of blindness), affecting 2.85 million (Mariotti, 2012). In addition to the global scarcity of donor cornea tissue, donor tissue rejection is a barrier to reducing blindness through keratoplasty. Transplants survival rates fall over time, presenting at ~ 90% within the first year and falling to ~40% by year 15 (Avadhanam, Smith, & Liu, 2015).
About our solution: The presented solution is an engineered corneal tissue: an acellular biosynthetic implant for corneal replacement or repair. Key advantages of the implant include its ability to: sustain critical quality attributes under ambient shipping and storage conditions, be surgically installed like donor tissue, sharply reduce the risk of immune rejection, and provide a more affordable and accessible option for cornea restoration.
Progress to date:
An early prototype of the implant is under development. Prototype development is a collaborative effort between Scigofer, LLC and Lehigh University. Current data shows that the implant supports cell growth, demonstrates transparency, and maintains structural integrity during suturing.
About Our Team

Creator: Amelia Zellander
Location: Pennsylvania
Bio: The company founder has a vision for expanding options for the repair and replacement of human tissues. Her career experiences include: 1) Over 5 years of industry R&D with medical device and pharmaceutical products, 2) Five years of academic research in tissue engineering, 3) Management and leadership roles in science and government.
Title: Founder, CEO
Advanced Degree(s): PhD
About Our Company
Scigofer, LLC
Location: 1758 Allentown Rd, #164
Lansdale, PA 19466
US
Founded: 2014
Product Stage: Prototype/MVP
Employees: 1-2
How We Help Physicians
The technology provides an alternative to donated cornea tissue that is highly accessible and has a room temperature shelf-life.
Innovation Details
Intellectual Property Summary
A provisional patent application is being prepared. The estimated date is by the end of May 2019. In the meantime, the technology has not been publicly disclosed and all exchanges of information for business purposes have been protected by non-disclosure agreements.
Clinical Information
This project is in the pre-clinical stage.
Regulatory Status
For the prospective licensor, the suggested FDA filing strategy is the 510(k) premarket submission as a class 2 medical device. The exit strategy includes licensing the technology following mature prototype completion.
How we will use the funds raised
The funding will support one year of pre-clinical prototype development. It will cover materials and wages for employees and contractors.
Thank You
This team has something to offer that may revolutionize corneal tissue restoration. Further, developments from this project can be leveraged to repair other tissue types. Medical breakthroughs come from the collective effort. We want to contribute to that collective effort, but we need your help.
Updates
No updates found .
Supporters
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.
Index Score
2
Score
0
Score
2
Likes0
Partners0
Pilots0
Follows-
This campaign has ended but you can still get involved.See options below.
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.



