BioAssistant Vitals: Wearable medical device for monitoring vital clinical data
by Muralidharan Gopalakrishnan
Wearable medical device for accurately monitoring advanced clinical insights on respiratory, cardiac, neural, SpO2 and other circulatory health patterns. It hardly requires professional supervision.
Boulder, CO United States Wearables Equity Raise WarOnCOVID challengeAbout our project

The problem we solve: Globally, there are more than 500 million people with cardiac, respiratory, neural and other circulatory health disorders. Continuous monitoring and regular health check-ups are necessary to reduce the risk of hospitalization and improve the health management experience of the patients with circulatory health issues. Conventional technologies for circulatory health assessments cannot cater to this need of the patients, since they are complex, prone to human errors, time consuming and requires constant professional supervision. Current wearable players are trying to develop a solution for this need, but again their technology is not of appropriate medical quality and only yield HR data prone to human errors. Owing to the current pandemic situation, medical centers are the last place for the patients with circulatory issues to spend time.

About our solution: We invented a compact multi-functional circulatory health monitoring wearable medical device to address this problem. BioAssistant Vitals provides error-free information and advanced clinical insights on the respiratory pattern, autonomous neural balance, blood oxygen data, cardiac rhythm and other medical condition. It is easy to use and human error-free medical technology hardly requiring any professional supervision. BioAssistant Vitals also comes handy for COVID-19 disease management.
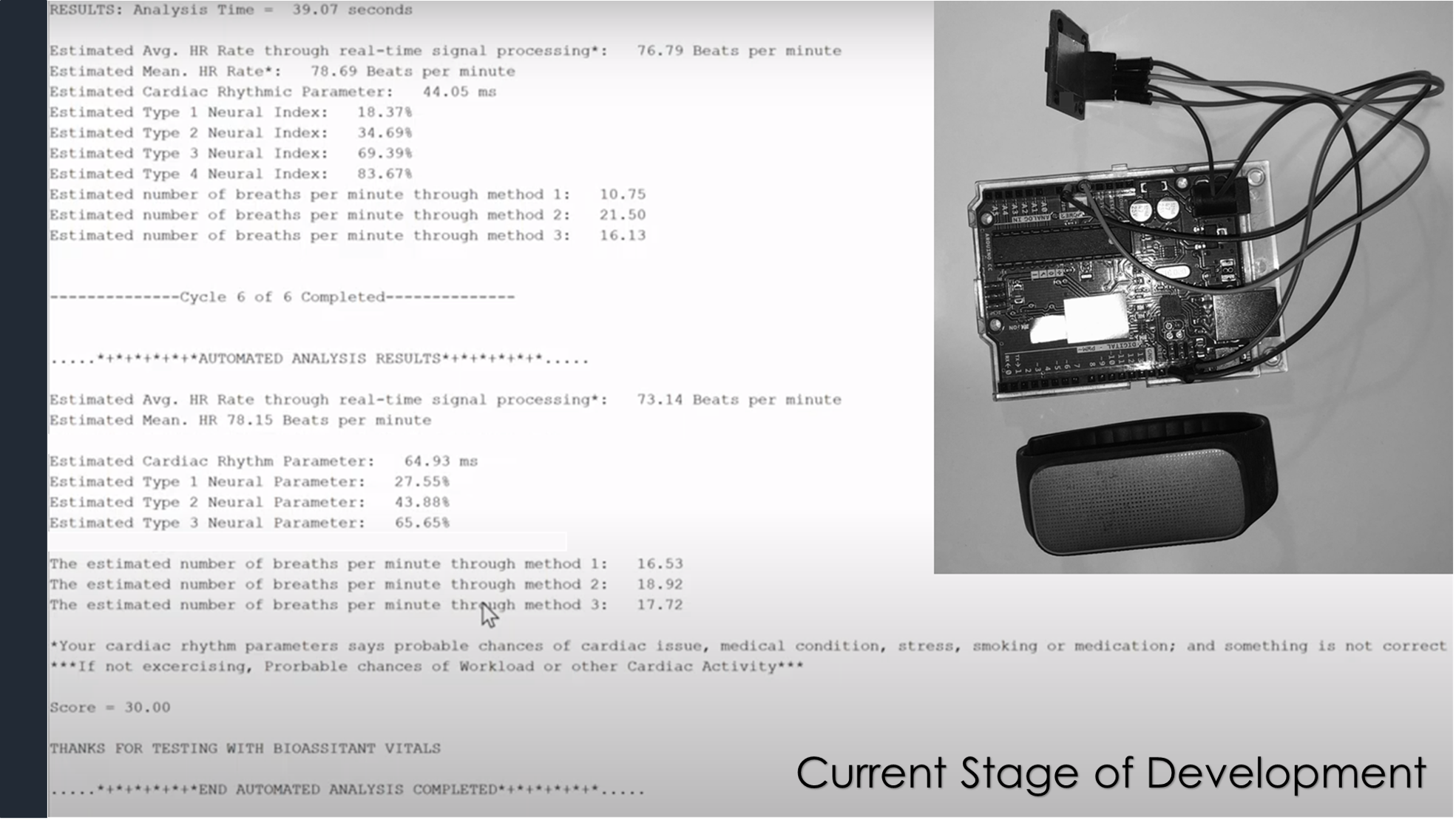
Progress to date:
- We invented the technology for accurately monitoring the respiratory pattern, autonomous neural balance, blood oxygen data, cardiac rhythm and stress signals. We built a real-time AI system to further diagnose the medical condition and to furnish advanced clinical insights. We also developed methods to overcome the movement and human errors, and now it can also even automically recognize a human sensing spot.
- We previously conducted a full-fledged sensor research to evaluate the accuracy and technology fit. Prior to the development, we filed the patents and secured other IP protection.
- We conducted live validation sessions with doctors from different medical background to evaluate our technology accuracy and use case. Every interviewed medical expert found the solution accurate and fit for their use.
About Our Team

Creator: Muralidharan Gopalakrishnan
Location: Colorado
Bio: Muralidharan is a techie with skills in Artificial Intelligence, Automation Technology, Medical Diagnosis Computational System Development, Hardware Design, Software Development, Embedded System, Intellectual Property and Lean & Agile. He has previous experience working on complicated medical research, AI and automation projects. He was a Management and Technical Advisory Consultant of Protiviti Middle East Member Firm, where he was working with top level management to help them re-design the management structure, process and IT security systems. He founded BioAssistant with vision to reshape the traditional medical diagnosis and healthcare practices.
Title: Founder
Advanced Degree(s): MS
About Team Members
Laurence Freeman
IP commercialization Partner and Business Mentor, SMC (Disp.) and FBDO.
Biography: Laurence runs a patent licensing company that has strategic relations with leading healthcare institutions across the globe. He has been advising BioAssistant on the business development aspects from the year 2017. He will be primarily be focusing on IP monetization and patent licensing.
Title: IP commercialization Partner and Business Mentor
Advanced Degree(s): SMC (Disp.) and FBDO.
Twitter:
@LAFreeman88
LinkedIn:
https://www.linkedin.com/in/laurence-freeman/
Surendiran G
Research Advisor, MD in Pediatrics
Biography: Dr. Surendiran is a paediatrician with knowledge in medical science and skills in some of the world's toughest medical treatment methods. He advises BioAssistant on the clinical research directions.
Title: Research Advisor
Advanced Degree(s): MD in Pediatrics
LinkedIn:
https://www.linkedin.com/in/surendiran-g-052b88105/
Pranav Kumar
Product Mentor, MBA in Marketing
Biography: He is Joint Secretary of the Infection Control Academy of India and runs a healthcare marketing consultancy. He has a vast experience working as chief experience officer for leading pharmaceutical companies.
Title: Product Mentor
Advanced Degree(s): MBA in Marketing
LinkedIn:
https://www.linkedin.com/in/pranavkr/
Thomas Shilling
FDA Mentor, MBA in F&A
Biography: Thomas is Director of Regulatory Affairs in the Advanced Engineering Management Company, a Denver based medical regulatory company. He has expertise in US medical regulations. He advises BioAssistant on the medical regulatory aspects.
Title: FDA Mentor
Advanced Degree(s): MBA in F&A
LinkedIn:
https://www.linkedin.com/in/thomas-shilling-14508a12b/
Gopalakrishnan Palani
Risk Mentor and F&F Investor, ICWA, MBA and B.Com
Biography: Gopalakrishnan was Ex Head of Internal Audit of Oil & Gas division of Reliance Industries Limited. He is an expert in risk management and Internal Audit. He infused the first round of capital for startup operations. He advises the company on the risk management aspects.
Title: Risk Mentor and F&F Investor
Advanced Degree(s): ICWA, MBA and B.Com
LinkedIn:
https://www.linkedin.com/in/gopalakrishnan-p-641b57b/
About Our Company
BioAssistant Ltd.
Location: 2718 Moorhead Avenue
Boulder, CO 80305
US
Founded: 2016
Website: http://bioassistant.org/
Facebook: https://www.facebook.com/bioassistant
Product Stage: Prototype/MVP
Employees: 1-2
How We Help Patients
We understand that you currently do have an efficient solution for monitoring your respiratory and circulatory health. We also understand most of the time you undergo false diagnosis is neither flaw in the doctors and current technology. This is why we invented a compact multi-functional medical device for accurately monitor your respiratory and circulatory health. Our solution is going to improve your diagnosis and clinical experience, since our technology has real-time AI system for providing advanced clinical insights on your respiratory pattern, autonomous neural balance, cardiac rhythm, blood oxygen and stress signals. You do not even have to worry about physically visiting the clinical center, since our medical device is free from human errors and it hardly requires any medical supervision. Now you can monitor your complete health at the comfort of your home.
How We Help Physicians
Most of the time your preliminary diagnosis becomes lengthy and inefficient is because you do not have the right kind of technology and data. This is where we come in to provide you a better preliminary diagnostic technology that can help you better assess your patients with any kind of circulatory health disorder. You do not even have to worry about physically monitoring your patients, since our technology enables remote diagnosis with more accuracy. You can comfortably spend your time where its creates a value for you. We will also provide automated clinical insights that you can analyze medical condition of the patient and to even detect the COVID-19 condition.
How We Help Hospitals
We understand that there is a heavy congestion in your centers and you are loosing your patients. With our technology, you do not have use the old methods that consumes the time and energy of both your doctors and patients. You can also use our technology for remote monitoring and it will literally increase your diagnosis capacity by 12X. This is the prefect time for you to adapt our technology, since we are in a pandemic crisis where your patients are afraid to visit the medical centers. Now you get increased patient capacity with lesser overhead costs.
How We Help Partners
- Wearable Health Tracker Companies: This is the opportunity for you to capture a very large market. We are going to provide you a fundamentally more efficient solution that is not only going improve your current accuracy, but more importantly will provide 5X more qualitative information. More accurate vital information and more in-depth analysis means more customers wanting to buy your product. We will also provide you with a COVID-19 tracking system and better sensing hardware. Your business will fly high and you do not even have to spend your reserves at this time on R&D.
- Medical Device Companies: This is perfect time for you to adopt our solution so that you can save your cost on the computational heavy system. Your system will now consume lesser computational and R&D time. Your system will never get struck and you do not even have to worry about the legal risks that comes with computational failure prone solutions. It is also a perfect replacement for many of your preliminary diagnostic devices, and you can keep the dedicated diagnostic machines for more detailed analysis. It is also a time where you need this solution, since your old human error prone technologies are not suitable for remote diagnosis. Trust me this is the time for the patients with COVID-19 or circulatory health problems not to visit the medical centers.
- Health Insurance Companies: We understand that you care about your customers and do not want any health concerns in the society. This is the best time for you to keep your circulatory health patients more safe, since they are the ones who are more likely to be affected by the current crisis. Help your customers adopt our solution right away and you will face lesser challenges in the future. It will also save your pay-outs. We are with you in your every pursuit of making the society healthier.
Challenge Mission
COVID Problem We Address
A COVID-19 patient generally experiences fast unsteady breathing, irregular cardiac activity, elevated stress levels, decreased oxygen capacity and disrupted neural activity. It is important to track these pathophysiological data to understand the criticality of the COVID-19 patient. If we can accurately track the health condition of a COVID-19 patient, then timely medical decisions can be taken. We have identified this problem and we are currently leveraging our invention to solve this problem.
Our COVID Solution
Our invention provides real-time information and advanced clinical insights on respiratory pattern, autonomous neural balance, cardiac rhythm, blood oxygen data and stress signals. A COVID-19 patient can wear our medical device to continuously track their pathophysiological condition. Doctors can use the data from our medical device to remotely track the clinical condition and the recovery progress of their COVID-19 patient. Our solution is also free from human errors and it hardly requires any professional supervision, hence it enables remote monitoring with more efficiency. Not only that non-COVID people with circulatory health disorders can also use our device as a preventive technology to get early COVID-19 warnings.
Innovation Details
Intellectual Property Summary
We filed 5 PCT patent applications on novel medical monitoring devices, which includes a totally non-invasive and painless solution for continuous glucose monitoring. We entered national phases several nations. We provided link to one of the patent applications.
Patent Link
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2019049116
Clinical Information
We conducted live validation sessions with doctors from different medical backgrounds to evaluate the accuracy of the technology and we also found success in it.
a) Few doctors conducted stethoscope tests to validate the accuracy of heart rate and breathing rate.
(Some doctors chose to use radial pulse measurement to evaluate these parameters)
b) Pulse oximeter tests were conducted to evaluate accuracy of the blood oxygen levels.
c) Some creative doctors chose to simulate the hypoxia condition by curtailing the oxygen supply to a particular sensing spot. They used the dropping oxygen levels as the indicators to conclude the accuracy.
d) Experiments were conducted on patients with medical history to evaluate the neural parameters.
e) To verify the accuracy of the neural patterns, it was also measured in stressful and exhausting work conditions. Some doctors who just came back from meditation tested it on themselves to evaluate the readings.
Regulatory Status
We are taking FDA Class 2 route to get clearance, since there are predicate devices and significant equivalents to validate the efficacy of the technology.
How we will use the funds raised
1. Product Development
2. Clinical Trials
3. Additional IP Filing and Protection
4. FDA Clearance
5. Pilot Stage Launch
Thank You
We are globally the first company to invent a fully automated comprehensive wearable medical technology for accurately monitoring respiratory pattern, autonomous neural balance, cardiac rhythm, blood oxygen data and stress signals. It currently has an in-built real-time AI system, which provides advacned clinical insights and in-depth information on the health condition of the patient. It hardly requires any professional supervision and it is free from human errors. We have procurement and licensing interests from leading healthcare companies. We filed patents to protect IP assets and this only one device among our product line. We also have intent for post clinical trials investment from several late stage VCs and a leading Indian tech corporate. This is one of the quickest time for us to get to the market and solve a big societal problem, so join us in this revolutionizing journey of reshaping the healthcare.
Investor Info
Market Size
The prevalence rate of circulatory health disorders is approximately more than 10%, thus there are more than 700 Million people across the globe with circulatory health disorders. 70 to 150 Million have the economic privilege and health insurance plans to buy one such medical device. Even if each device is sold at USD 200, then the total obtainable market is ~ US$ 20 - 30 Billion.
Projected 3 Year Growth
Assumptions
Year 0: Completion of product development, clinical trials, medical approval and pilot launch
Year 1: Series B Fundraise and 5% market penetration.
Year 2: Series C Fundraise and 15% market penetration.
Year 3: Series D/E Fundraise and 25% market penetration.
Revenue Projection
Year 0: USD 0
Year 1: USD 700 Million - 1.5 Billion
Year 2: USD 2.1 - 4.5 Billion
Year 3: USD 3.5 - 7.5 Billion
Parallel Revenue Streams:
We also intend to licensing our technology, and it will separately generate yearly licensing revenue and royality revenue. This revenue could be in the range of few hundreds of millions of dollars.
Revenue Model
Revenue Streams
1. IP Licensing to wearables health tracker companies
2. IP Licensing to medical device players
3. Direct Sales in the places where people are economically less previleged
Competitors
The competitive advantages that we have that other players in this market do not understand
1. Accuracy is first and foremost priority of any medical professional that many competitors do not understand. Many doctors told me that they do not want to use current solutions since they are not reliable and test results often change.
2. Multitude of vital clinical information is essential to correctly diagnoise and treat a patient with circulatory health issues.
3. Speediness of the diagnosis solution is very important to boost the businesses of the medical diagnostic chains and medical centers, since it increase their diagnosis capacity.
4. Home based remote monitoring solution should be human error free, since you cannot translate a complicated error-prone solution for such a use case.
5. Functionality is very crucial to understand the solution and raw data does not do any good, since most of the users get confused with just raw data.
Competitors
1. Medtronics
2. Masimo
3. Philips
4. Siemens
5. Cardiogram
6. Oxitone
7. Isansys Lifecare
8. Current Health
9. iBeat
10. AliveCor
11. Apple
12. Fitbt
13. Samsung Gears
14. Withings
15. Xiaomi
16. Huawei Technologies
Traction
Three leading medical chains in India and several medical doctors were impressed with the solution and expressed their interest in procuring our medical devices. One of them is Medall, which is second largest medical diagnostic chain of India. Another one is Meddo, who runs around 500 medical centers across India. We also had talks with various health insurance companies who said that they might be interested in working with us.
Due Diligence Docs
Please note that access to the company's confidential materials is limited. Click this button to request access from the Company and its representatives.
Updates
No updates found .
Supporters
-

07/03/2020 - Liked the project.
06/23/2020 - Followed the project.
06/23/2020 - Liked the project. Instant Feedback
Instant Feedback
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.
17Medstartr
Index Score17
Interest
Score0
Adoption
Score3
Likes0
Partners0
Pilots2
Follows-
This campaign has ended but you can still get involved.See options below.
$ 1,500,000 goal
Instant Feedback
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.

