PONS: Re-inventing pre-hospital diagnostics of brain and spine injurie
PONS is an affordable mobile diagnostics tool for brain/spine injuries by utilizing ultrasound technology, in order to provide first responders a tool that they can use on the field
Jersey City, NJ United States Diagnostics 2021 Vision challengeAbout our project

The problem we solve: Today, ambulances lack an early recognition device that is connected to hospitals that EMTs can use to detect traumatic brain injuries. Patients instead must wait on average [60] minutes until they get to a hospital, at which time a doctor runs through a triage checklist to determine if there might be a TBI. The potential for mis-diagnosis is high given non-presenting symptoms and doctor expertise. Cost concerns also come into play as brain scans are exorbitantly expensive. At this point if diagnosed with a TBI, the patient is taken for a CT scan, where they may have to wait further depending on availability and order of criticality compared to other patients. The compounding effect of lost time and diagnosis error contributes to the over 53,000deaths annually in the US from TBIs and the more than 5.3M(Source: CDC) Americans currently living with long-term disabilities directly attributable to TBIs.
About our solution: PONS is a patent-pending machine learning approach for the automatic identification and risk categorization of traumatic brain injuries and spine injuries by using ultrasound (US) scans to detect anatomical brain features. Delivered as an easy-to-use and mobile prediagnostic tool, PONS enables any qualified medical professionals in the field in seconds to assess the probability of a TBI, saving critical time that could mean the difference between a patient's full recovery or unnecessary death
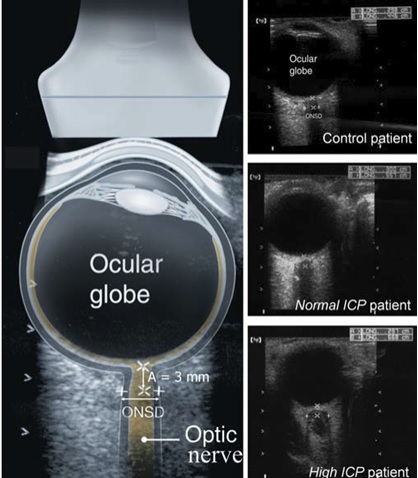
Progress to date:
We have expertise in the area of deep learning, algorithm development, and application of ultrasound imaging for various surgical and non-surgical procedures. We have identified a unique need that is not addressed currently: A portable, noninvasive, data-driven imaging solution for on-site TBI assessment.
The research of PONS has started 3 years ago, we have done several clinical trials as of today. Because of COVID, the clinical trials have stopped. Our road map is to get FDA approval by the end of 2021 and lunch the product.
Data is the new oil in AI-based health care technology. Currently, we are in the process of collected ultrasound data from healthy as well as individuals who are diagnosed with TBI. Once the data collection is finalized we will train our previously developed deep learning methods on the collected data.
The main objective of PONS is validation, and deployment of new computational tools, based on deep learning, for processing multi-feature ultrasound data to derive models that yield individual-level accurate risk assessment and TBI diagnosis and monitoring. Our solution is to operate on a standard point of care ultrasound probe. Therefore, our solution provides a clear path for fast adaptation. Due to its compact nature, our proposed solution also lends itself to repeated bedside imaging studies for monitoring TBI progression which is crucial to adjust the treatment plan. The use of point of care ultrasound for TBI patient risk assessment and treatment response monitoring has not been investigated so far. We will collect ocular ultrasound and transcranial ultrasound data. B-mode (grayscale intensity-based) ultrasound data, radio frequency (RF) raw ultrasound signal data, and local phase tissue signatures will be treated as multi-feature data. Such an integrative data collection using ultrasound in the context of TBI diagnosis, risk assessment, and monitoring is currently not available.
About Our Team

Creator: soner hacihaliloglu
Location: New Jersey
Bio: Soner Hacihaliloglu started his professional career as Project Manager at Siemens Ares-ecount Technologies. After working 3 years in Building Technologies he was appointed as the MENA Region Business Development manager at E-on Ista Holding. During the time he served as a Business development manager he was responsible for the operations in 5 different countries on 3 continents. He set up operations from 0 to 50m Euro revenue in 2 years. Soner has been chosen as one of the best Innovators under 40 and the Best young Energy Professional in the MENA region in 2017 and 2018. As of 2020, Soner is the CEO of PONS Technologies, a company that has been awarded by the UN, Europe Union, Sustainable Management Assotioains as one of the best innovations Soner has a Computer Science Degree from California University Newport and an MBA degree from Erasmus University Rotterdam School of Management
Title: CEO / Co-founder
About Team Members
Ass.Prof.Dr. Ilker Haci
CTO, PhD, Prof
Biography: Ass. Prof. Dr. Ilker Hacihaliloglu is the director of the Computer-Assisted Surgery and Therapy Laboratory (CompAST) at Rutgers University’s multidisciplinary research laboratory focusing on developing non-robotic surgical systems. He has over 15 years of experience in computer-aided surgery and early diagnostics systems in the brain, cancer treatment fields.
Title: CTO
Advanced Degree(s): PhD, Prof
About Our Company
PONS
Location: 206 Warren St
Apt 1L
Jersey City, NJ 07302
US
Founded: 2020
Website: https://www.ponstech.co
Twitter: @ponstech
Product Stage: Idea
Employees: 1-2
How We Help Patients
PONS will provide a mobile and easy to use early recognition technology to situations or geographies where high tech health devices can’t be used or bought. PONS will establish itself as a platform for image capture and recognition. By enabling experienced doctors all around the world with PONS technology, we can get one step closer to providing more equitable health care, even if you don’t live in developed countries. The PONS product and R&D road map are focused on converting current US probes into wearable devices that will free up doctors to use their hands and to analyze the potential brain bleeding risks with the help of machine learning to categorize those risks.How We Help Physicians
Current traditional brain health diagnostic procedures, like MRI and CT, are time-consuming, emit radiation, expensive, and access to the equipment and the results are time-delayed (i.e. 30-70 minutes). Importantly, patients have to be transported (sometimes over long distances) and admitted to a hospital or specialized medical center (that has the equipment and highly qualified technical and medical personnel) before they can be diagnosed with a Brain Trauma. The medical industry is beckoning a quick, reliable, and less expensive brain trauma diagnostic solution. By using PONS, allowing doctors to remotely advise paramedics treating patients at the scene or in transit. PONS integrated ambulances will improve the speed of treatment, reducing the number of patients taken to hospital and relieving strain on hospital ER resources. next-generation connectivity could free-up 10.1 million hours per year for the hospitals as well as saving cities $1B per year and decreasing overall bed occupancy rates by 6% through the adoption of wearing monitoring devices.How We Help Hospitals
Head Injury (HI) is a leading cause of death and disability among the predominately young population. Ten million cases of HI are estimated per year worldwide. Annually, within the United States, there are about 2 million emergency room visits for HI, roughly 575,000 admissions for brain trauma, nearly 52,000 deaths, and approximately 80,000 cases of severe long-term disability.1 About 10% of combat injuries sustained during conventional land warfare involve HI, including skull and brain traumas. The estimated overall burden of TBI on the US economy was approximately $76.5B(CDC), with costs for disability and lost productivity outweighing those for acute medical care and rehabilitation. The annual costs of TBI care are over $400B according to the study of the European Brain Injury Consortium. TRAUMATIC BRAIN INJURY (TBI) causes more death and disability than any other trauma-related injury and affects an estimate of 69 million people worldwide each year. an estimated 69.0 million (95% CI 64.2–73.8 million) people worldwide will suffer TBI each year. PONS will help to reduce overcrowding in the ER and reduce the cost of unnecessary use of CT and MRIs by 25%How We Help Partners
Current traditional brain health diagnostic procedures, like MRI and CT, are time-consuming, emit radiation, expensive, and access to the equipment and the results are time-delayed (i.e. 30-70 minutes). Importantly, patients have to be transported (sometimes over long distances) and admitted to a hospital or specialized medical center (that has the equipment and highly qualified technical and medical personnel) before they can be diagnosed with a Brain Trauma. The medical industry is beckoning a quick, reliable, and less expensive brain trauma diagnostic solution. Detect + Quantify + Predict The next stage in the evolution of AI solutions for medical imaging is expected to be the addition of predictive analysis, in addition, to detect and/or quantify. PONS solutions offer real-time diagnostic decision support, such as the likelihood of malignancy (following lesion/nodule detection and quantification), or a differential diagnosis (based on a confidence score), leading to earlier detection and diagnosis. PONS solutions will enable doctors, surgeons, field medics, and radiologists to prioritize their focus on cases of greater clinical need and to determine with greater confidence whether follow-up invasive procedures (for example, biopsy) are required. Although the final diagnosis will require the radiologist’s or doctor's own judgment, predictive analysis will greatly enhance the value of AI solutions to doctors, surgeons, field medics, and radiologists both inside and outside the hospital.Innovation Details
Intellectual Property Summary
Various groups have investigated the use of ultrasound for the assessment of intracranial pressure from optical scans. However, current methods are not accurate and require manual interaction. Our technology is fully automated and is based on the use of multi-feature ultrasound data acquired from the patient. Multi-feature data will be collected from traditional B-mode ultrasound data, raw (radio-frequency) ultrasound data, image phase information. Such a multi-feature platform is currently not available. Our group has extensive experience in developing artificial intelligence solutions for various applications where multi-feature ultrasound is applied which put us in a unique position to succeed in this project.
The artificial intelligence platform and the head-mounted imaging system will be patentable.
Clinical Information
FDA has recently proposed a regulatory framework for modifications to Artificial Intelligence/Machine Learning (AI/ML)-Based Software as a Medical Device (SaMD). We will follow these guidelines and have discussions with the FDA during this process.
https://www.fda.gov/media/122535/download
Regulatory Status
We will start the clinical trials in 2021 and after 200 trials we will apply for FDA approval. Our system is classified as Class 2 devices, meaning they are non-invasive and the track to FDA approval is faster than usual.
Our strategy is to develop partnerships with research universities and hospitals so that we can accelerate the process both on clinical trials but also on FDA approval.
How we will use the funds raised
Our plan to use of the funds is as follows
40% R&D
30% Sales and marketing
30% legal and certification.
Thank You
We are developing an affordable mobile diagnostics technology by utilizing ultrasound technology. The system is meant to be used by doctors who serve in difficult circumstances and geographies like 3rd World countries, villages, immigration centers. Doctors without borders, Red Cross, UN, are some of the targets groups that we want to reach and provide a smart and affordable diagnostics tool that can be used to track and monitor brain, spine injuries where expensive and big systems can not be used.
We want to reach out to 1000 doctors that are working in the field. According to the studies of the UN and Accenture 5 Billion people dont have access to smart surgery and diagnostics systems in health care. Every year we will spend by 2030 over USD 12.3 Trillion because of the lack of early diagnostics and surgery systems. We want to reduce that amount by 25%
Updates
No updates found .
Supporters
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.
Index Score
13
Score
0
Score
1
Likes0
Partners0
Pilots0
Follows-
This campaign has ended but you can still get involved.See options below.
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.



