SecureCHEK AI : Smarter and Safer Rx Marketing with AI
We're fixing marketing inefficiencies in life sciences—saving time and money while ensuring healthcare professionals, patients and payers get accurate, balanced info about medicines
New York, NY United States AI / ML Equity RaiseAbout our project
The problem we solve: In Life Sciences, marketing materials face slow, error-prone compliance reviews that can drag on for weeks or months. Drug and medical device companies hire communications agencies to prepare product materials to educate and promote their products. These agencies manually evaluate these materials prior to review by the Medical, Legal and Regulatory (MLR) team to ensure they are ready for approval. However, manual processes introduce mistakes that must be fixed by expensive MLR subject matter experts —wasting time, driving up costs, and delaying critical information about prescription products from reaching HCPs, patients and payers. These delays hurt launch velocity, sales, and patient outcomes. The current system isn’t just inefficient—it’s broken. The need for automation is urgent.
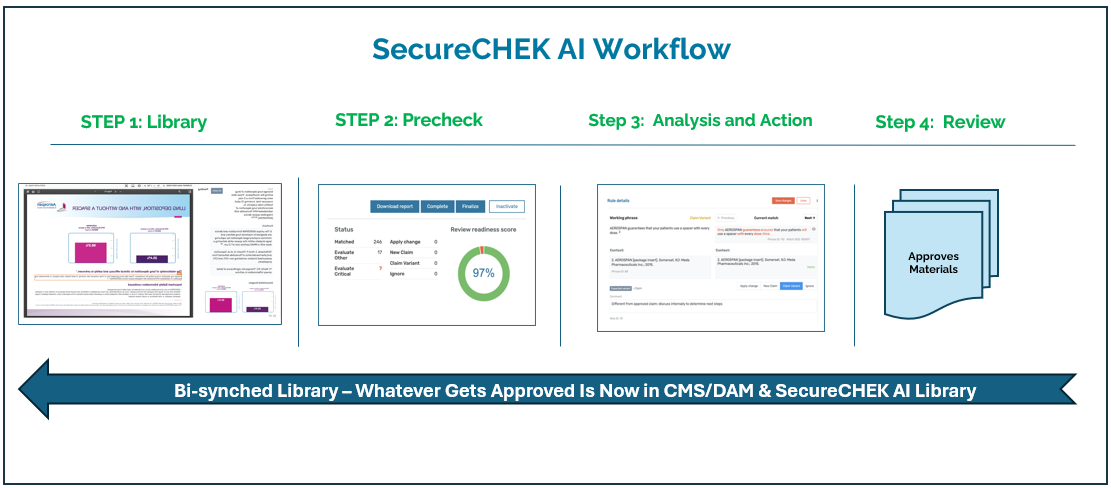
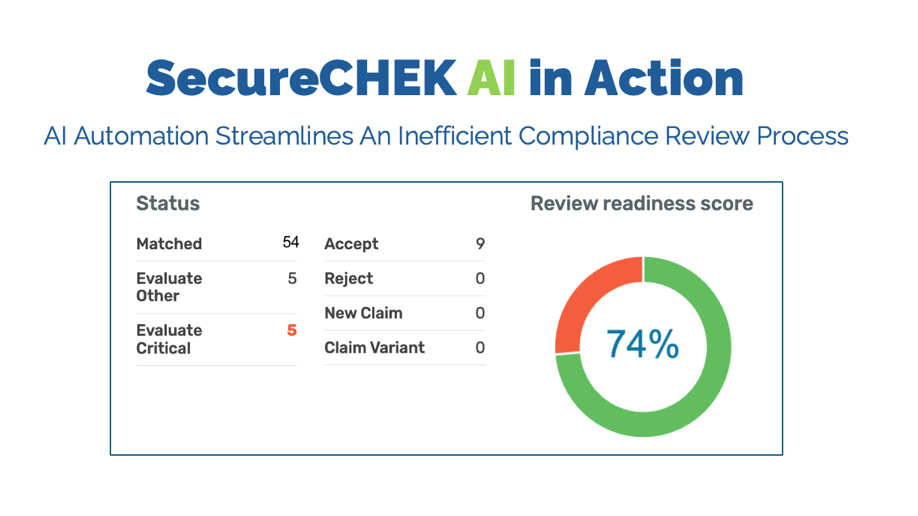
About our solution: SecureCHEK AI solves a costly, time-critical problem in Life Sciences: the slow, manual review of regulated marketing content. Our proven SaaS platform uses a powerful combination of Analytical and Generative AI to flag compliance risks—so medical, legal, and regulatory teams spend less time reviewing and more time approving. By verifying scientific accuracy and medical integrity, SecureCHEK AI helps ensure that HCPs, patients and payers make informed decisions, which is central to every government agency’s public health mandate. The software prevents risky communication by identifying omissions of risk information, overstatements of efficacy, and unsubstantiated comparisons. The result: materials get to market faster, unlocking commercial impact with launch velocity and helping patients and providers access critical drug information sooner.
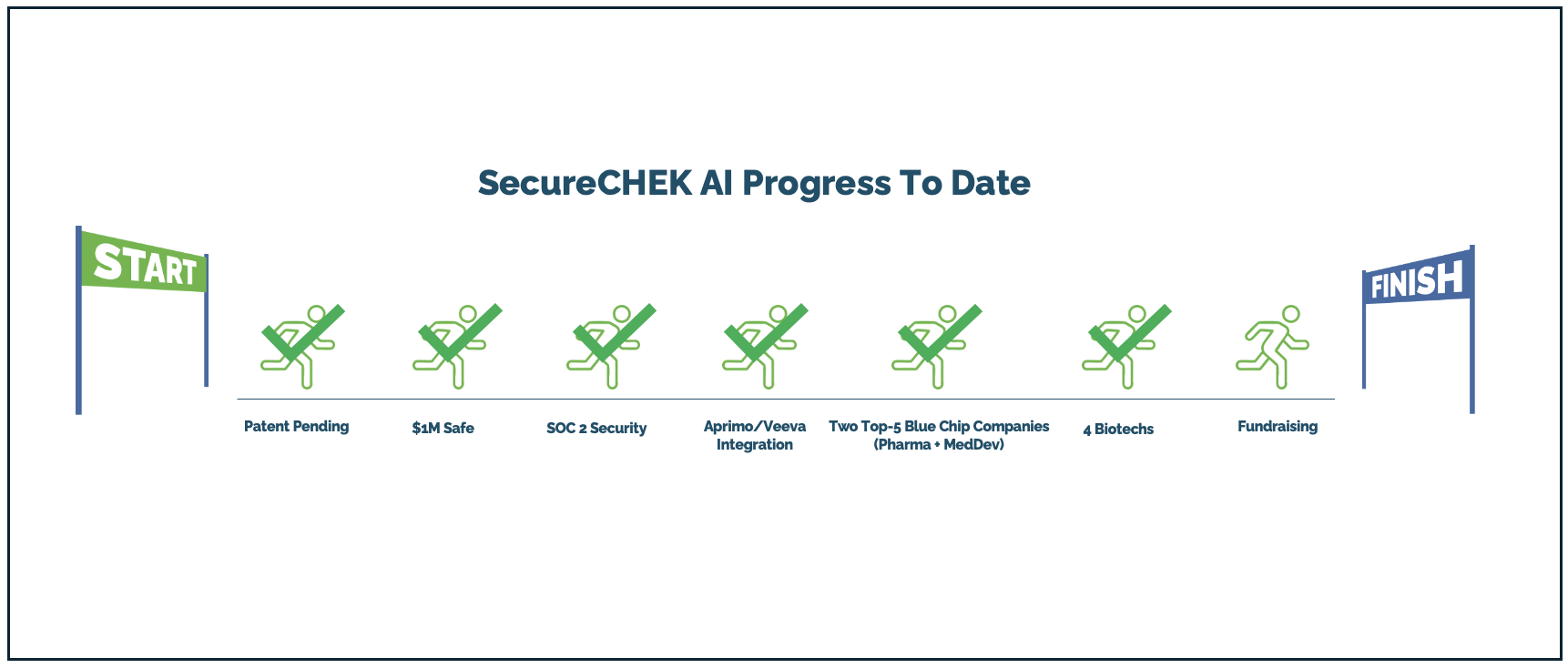
Progress to date:
SecureCHEK AI is in market and scaling. We are the first AI-powered prechecking tool for Life Sciences that combines Analytical and Generative AI to accelerate compliance reviews. We’re integrated with Veeva and Aprimo—the two leading compliance workflow platforms—enabling faster adoption through trusted channels. Aprimo alone drove $1M in licensing revenue. Current clients include a top 5 pharma company, a top 5 medical device company, and four onboarding biotech firms. Our solution is live, delivering measurable ROI, and earning strong validation across the industry .
About Our Team

Creator: Ilyssa Levins-Pimienta
Location: New York
Education: NYU
Bio: Ilyssa is an entrepreneur who challenges the status quo with cutting-edge solutions for life sciences. She is responsible for a series of “firsts” in the industry beginning with her career at a global healthcare marketing agency, where she launched and ran several healthcare communication agencies for Grey and WPP. This led Ilyssa to launch the Center for Communication Compliance in 2008, an online training and certification company that helps marketers and their agencies effectively promote prescription drugs in compliance with government laws and policies. The CCC curriculum is currently offered by the Biotechnology Innovation Organization (BIO). Ilyssa then created a prototype for her original idea to precheck materials prior to review and approval. Once AI became accessible to the public, Ilyssa decided to embark on her next venture culminating in the launch of SecureCHEK AI. Ilyssa received the President Award from the Healthcare Businesswomen's Association for conceiving and launching the industry’s 1st Certificate Program for Digital Innovation. Ilyssa was named one of the industry's 100 Inspiring People by PharmaVoice Magazine.
Title: CEO
Advanced Degree(s): BA
About Team Members
Michael Levins
President, BA
Biography: Michael is a seasoned entrepreneur with a proven track record of founding, scaling, and exiting multiple companies across global markets. He brings deep operational expertise and strategic vision to SecureCHEK® AI, where he is currently focused on scaling the company’s operations, building a high-performance team, and executing a growth plan in the life sciences compliance technology space. Prior to launching SecureCHEK® AI, Michael founded and led several successful ventures. His first company, Innovative USA, a contract manufacturing enterprise, operated international offices in Taiwan and Hong Kong, manufactured in five countries, and served a global customer base. He later founded a children’s publishing company, InnovativeKids, which developed over 400 titles, sold through 2,500+ retail locations, and was translated into more than 14 languages. The company held more than a dozen patents and was named one of Working Mother Magazine’s “Best Companies to Work For.” After a successful exit, Michael co-founded a direct-to-consumer activewear brand that achieved nationwide and international sales and now operates independently. Throughout his career, Michael has demonstrated an exceptional ability to identify emerging market opportunities, create valuable intellectual property, and build sustainable enterprise value. He holds a BA from Columbia University.
Title: President
Advanced Degree(s): BA
LinkedIn:
https://www.linkedin.com/in/michael-levins-7b68274/
Cindy Machles
Chief Marketing Officer, BA, MBA
Biography: Cindy is known for disrupting the way the pharmaceutical industry does business. With her experience as brand manager at Revlon, Clorox, and Johnson & Johnson, Cindy became one of the first advertising executives to apply marketing principles from consumer-packaged goods to healthcare. Cindy founded a global strategy practice for the WPP/GHG agency and became Global Practice Leader, successfully raising the standards of strategic thinking and delivery for the agency around the world. Cindy, the co-founder and CEO of Glue Advertising, was recognized as a top 100 innovator and entrepreneur (2022). Her trailblazing activities on women’s issues were featured in two books in 2021—Surrounded by Awesome Women and Founded by Women: Inspiring Stories from 100 Female Founders. She is a co-head of Chicago Booth Angels Network and an advisor to young companies within University of Chicago’s prestigious accelerator, The Polsky Center. Cindy has a BS summa cum laude from Boston University and an MBA from University of Chicago’s Booth School of Business.
Title: Chief Marketing Officer
Advanced Degree(s): BA, MBA
LinkedIn:
https://www.linkedin.com/in/cindy-machles-6b7b944/
Misa Petrovic
Chief Product and Technology Officer, Electrical Engineering, Computer Science
Biography: Misa is a seasoned software architect and AI technology leader with over 20 years of experience building enterprise applications across cloud, AI, and content management domains. He currently serves as Chief Product & Technology Officer at SecureCHEK® AI, where he leads the development of an AI-powered SaaS platform that accelerates regulatory (MLR) review processes for life sciences companies. His work bridges deep AI/ML innovation—including GenAI, RAG, and NLP—with enterprise-grade reliability and compliance. Misa is also the Principal Consultant and Head of the Cloud & AI Practice at S4HC, where he has spearheaded engineering efforts on cloud-native solutions using AWS, GCP, Kubernetes, and containerized architectures. His technical leadership spans sectors including healthcare, life sciences, telecommunications, and finance, with extensive experience integrating and customizing enterprise content management systems such as Alfresco. He has architected and led engineering on high-impact software products, including SecureCHEK AI, AI-powered medical case review tools, and complex ECM deployments for global brands. Misa holds a postgraduate certificate in Computer Science from ETH Zürich and an Electrical Engineering degree from the University of Belgrade. His rare combination of deep technical acumen and product leadership makes him a critical driver of scalable, compliant, AI-first innovation.
Title: Chief Product and Technology Officer
Advanced Degree(s): Electrical Engineering, Computer Science
LinkedIn:
https://www.linkedin.com/in/milivoje-petrovic-b163541/
About Our Company

SecureCHEK AI
Location: 303 E 57 street
21B
New York, NY 10022
US
Founded: 2021
Website: https://securechek.ai/
Product Stage: In the Market
Employees: 3-5
How We Help Patients
SecureCHEK AI helps patients in the following ways:
Empowers Decision-Making: Patients can better understand the benefits, risks, and limitations of a treatment, enabling them to make decisions that align with their values, preferences, and health goals.
Builds Trust: When information is transparent and unbiased, patients are more likely to trust their healthcare providers and the healthcare system as a whole.
Improves Adherence: Knowing what to expect—including potential side effects and how to manage them—can increase a patient’s confidence in starting and continuing treatment as prescribed.
Promotes Safety: Accurate information helps patients recognize warning signs, avoid harmful drug interactions, and use the medication properly, reducing the risk of misuse or adverse events.
Supports Shared Decision-Making: Balanced education enables patients to ask meaningful questions and engage in informed conversations with their providers, resulting in more personalized care.
How We Help Physicians
SecureCHEK AI helps providers as follows:
Supports Evidence-Based Practice: Reliable, unbiased data enables HCPs to make clinical decisions rooted in science, improving patient outcomes and maintaining professional standards.
Improves Treatment Selection: Clear understanding of benefits, risks, indications, and limitations helps clinicians choose the most appropriate therapy for each individual patient.
Enhances Patient Communication: Accurate information equips HCPs to educate patients effectively, address concerns, set realistic expectations, and foster adherence.
Reduces Legal and Ethical Risk: Using only balanced and substantiated information protects providers from inadvertently misleading patients or violating regulatory standards.
How We Help Hospitals
SecureCHEK AI helps hospitals as follows:
Enhances Patient Safety and Outcomes: Ensuring staff have access to reliable drug information reduces the risk of medication errors, adverse events, and inappropriate prescribing, improving overall patient care.
Supports Clinical and Operational Decision-Making: Accurate product information helps pharmacy and therapeutics (P&T) committees, formularies, and care teams make informed decisions about which drugs to approve, stock, and prioritize.
Promotes Regulatory and Legal Compliance: Hospitals must meet strict FDA, Joint Commission, and CMS standards. Using truthful, balanced drug information helps maintain compliance and avoid costly violations.
Builds Trust with Patients and Providers: When hospitals communicate clearly and transparently about medications, they build credibility and trust, which strengthens patient satisfaction and provider confidence.
Drives Efficiency and Cost-Effectiveness: Balanced information helps identify the most appropriate and cost-effective therapies, supporting stewardship programs and reducing unnecessary utilization.
How We Help Partners
SecureCHEK AI offers partners in life sciences a direct path to solving one of the industry's most urgent and costly challenges: ensuring promotional and scientific content is accurate, compliant, and audit-ready—without slowing down time-to-market. Our innovation directly supports pharmaceutical, biotech, and medical device companies by reducing manual effort, increasing confidence in regulatory submissions, and minimizing the risk of non-compliance.
By integrating seamlessly with industry-standard platforms like Veeva and Aprimo, SecureCHEK AI fits naturally into existing Medical, Legal, and Regulatory (MLR) workflows. This means partners don’t need to overhaul their systems—they just plug in our technology to gain immediate value.
A pilot or strategic partnership with SecureCHEK AI offers clear and measurable benefits:
-
Accelerated content approvals
-
Reduced compliance risk and rework
-
Improved internal alignment across medical, legal, and marketing teams
-
Greater trust from regulators, healthcare providers, and patients
Ultimately, we make our partners more successful by helping them scale safely, communicate confidently, and operate more efficiently in a highly regulated space. We welcome partnerships with companies who are serious about solving real problems—and we believe in fair value for meaningful collaboration.
Innovation Details
Intellectual Property Summary
The SecureCHEK AI patent application was published Jan 2, 2025. It describes a software-based AI system that automates the review of regulatory content in highly regulated industries like pharma and medical devices. It improves speed, accuracy, and consistency in processes such as Medical, Legal, Regulatory (MLR) review by identifying errors, inconsistencies, or omissions in promotional or scientific materials before human review.
Clinical Information
We are not a drug or device
Regulatory Status
We are not a drug or device
How we will use the funds raised
SecureCHEK AI is raising capital to strengthen our leadership position and accelerate growth across three key areas:
-
Research & Development (53% of the round):
A majority of the funds will be invested in enhancing our core technology platform. This includes advancing our proprietary AI models to further improve accuracy, scalability, and speed in identifying inaccurate product claims across regulatory, legal, and medical review processes. These investments are essential to stay ahead of competitors, maintain our IP advantages, and address evolving compliance requirements in life sciences communications. -
Sales, Marketing & Customer Acquisition (27% of the round):
To drive top-line growth, we will allocate funds toward expanding our go-to-market efforts. This includes hiring and training a specialized sales team, launching targeted marketing campaigns, attending key industry events, and strengthening brand visibility. These efforts will support customer acquisition, increase market awareness, and position SecureCHEK as the preferred AI partner for compliance and promotional review. -
Operational Infrastructure & Scaling (20% of the round):
The remaining portion of the capital will support scaling our business operations to ensure we can serve a growing number of enterprise clients efficiently and securely while maintaining service excellence.
Thank You
Join us in transforming how critical information about prescription products reaches HCPs, patients and payers quickly, accurately and truthfully. SecureCHEK AI accelerates Medical, Legal and Regulatory (MLR) compliance reviews, eliminating costly bottlenecks that prevent fairly balanced content from reaching providers, patients and payers. Life Sciences is an innovation-driven sector facing rising content volume, regulatory complexity, and pressure to cut costs. The need for automation is urgent. Our AI platform meets this moment—and the market is ready. Let’s lead this disruption together.
Investor Info
Market Size
SecureCHEK AI is targeting the global life sciences industry, which allocates over $100 billion annually to marketing and promotional activities. Approximately 9% of this spend—roughly $9 billion—is directed toward categories directly aligned with SecureCHEK AI’s capabilities, such as promotional review, regulatory compliance, and medical content validation. Our estimated Total Addressable Market (TAM) is $2.5 billion, based on our target segments and serviceable functions.
There are over 11,000 marketed prescription products globally across pharmaceuticals, medical devices, and veterinary medicine. Each product typically requires support across multiple functions, including commercial promotion, medical affairs, and investigational use in clinical research. Based on licensing fees for core platform functionality, value-added services, and premium capabilities, our projected annual spend per client is approximately $230,000 per product. This pricing reflects the high regulatory stakes, the complexity of the review process, and the cost savings clients can realize by reducing risk, cycle times, and manual effort.
Projected 3 Year Growth
SecureCHEK AI is projecting steady and strategic revenue growth over the next three years, with a strong emphasis on building long-term enterprise value. In 2025, the company expects to generate $0.9 million in revenue, reflecting a focus on customer onboarding, pilot testing, and initial market penetration at introductory pricing levels. Revenue is anticipated to grow to $2.5 million in 2026 as early adopters convert to paying customers and additional pilots are brought online. During these early years, gross margins are expected to be approximately 65%, accounting for higher support and implementation costs typical of early-stage SaaS deployments in regulated industries.
By 2027, SecureCHEK AI anticipates a significant inflection point, with revenue reaching $12.5 million. This growth is driven by the transition to full-scale commercialization and expansion across pharmaceutical, medical device, and veterinary markets. Gross margins are projected to improve to 75% as the company benefits from increased automation, standardized onboarding,, operational efficiencies and a strong customer referral pipeline.. This margin improvement signals a scalable model with strong unit economics, laying the foundation for long-term profitability and enterprise value.
Revenue Model
SecureCHEK AI is a subscription-based SaaS platform with annual licensing fees tied to the number of brands supported. This scalable, modular pricing model creates highly predictable recurring revenue and positions us for significant expansion within each client organization. Most life sciences companies manage dozens of commercial and pipeline brands, providing ample opportunity for conversion over time. Adding additional products to an existing client account is virtually costless from an infrastructure standpoint, allowing gross margins to scale to 90–95% per license.
Our land-and-expand strategy begins with a focus on high-priority, promoted products, then extends into pre-commercial and investigational brands. The platform’s high stickiness comes from its deep integration into clients’ medical, legal, and regulatory (MLR) review workflows, content approval systems, and compliance protocols. Once embedded, SecureCHEK AI becomes central to daily operations—making replacement both technically disruptive and operationally risky.
Clients are often reluctant to consider alternative solutions after implementation due to the high switching costs, the need for cross-functional retraining, and the complexity of integrating a new system into established review and compliance processes. This entrenched position not only enhances long-term retention but also drives sustained account growth and maximizes customer lifetime value.
Competitors
SecureCHEK AI is redefining how life sciences companies manage compliance and accuracy in their promotional and scientific content. Our platform uniquely combines the power of Analytical AI and Machine Learning with the creative flexibility of Generative AI to deliver a next-generation prechecking solution that is not only fast and efficient, but also verifiable, audit-ready, and deeply reliable.
While most competitors rely solely on GenAI—which excels at tasks like summarization, drafting, and Q&A—it also comes with a critical flaw: the tendency to produce factually incorrect yet convincing content, known as hallucinations. In regulated industries like life sciences, where misinformation can lead to serious regulatory, legal, and reputational consequences, this is simply unacceptable.
SecureCHEK AI eliminates that risk. Our hybrid architecture ensures that every insight is grounded in structured, validated data while still offering the usability benefits of GenAI. The result: higher accuracy, greater transparency, and significantly reduced compliance risk.
By addressing the industry’s top concerns—data security, regulatory confidence, and factual integrity—we give our clients a clear advantage: a trusted, scalable solution they can confidently integrate across their product portfolios.Please tell us about your current interest and sales (traction) with customers and partners or potential customers if you have not finished your product yet.
Traction
SecureCHEK AI is already integrated with the two largest compliance workflow platforms in the life sciences industry—Veeva and Aprimo—positioning us at the core of enterprise MLR operations. This seamless integration accelerates adoption, reduces friction, and makes us plug-and-play for top-tier pharmaceutical and biotech organizations.
We’re proud that a top five medical device company, a top five pharmaceutical company are among our clients, validating both the scalability and credibility of our solution. In addition, we are actively onboarding four innovative biotech companies, signaling strong momentum and demand across both established enterprises and emerging players.
These milestones not only demonstrate market traction but also reinforce SecureCHEK AI's position as a trusted, enterprise-ready partner in regulated AI adoption.
Due Diligence Docs
Please note that access to the company's confidential materials is limited. Click this button to request access from the Company and its representatives.
Updates
No updates found .
Supporters
-

09/10/2025 - Liked the project. Instant Feedback
Instant Feedback
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.
3Medstartr
Index Score3
Interest
Score0
Adoption
Score2
Likes0
Partners0
Pilots1
Investors-
This campaign has ended but you can still get involved.See options below.
$250,000 pledged of $2,500,000 goal$250,000 Investor, Pilot & Parnter
interest to date.
Instant Feedback
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.


