Spinal Cord Repair with Cells!: Expediting a Promising Stem Cell Treatment for Quadriplegia
Accelerate the clinical translation of a patient-specific stem cell breakthrough, proven to regrow spinal cord tissue in a quadriplegic man, that is going into clinical trial.
New York, NY United States Regenerative MedicineAbout our project
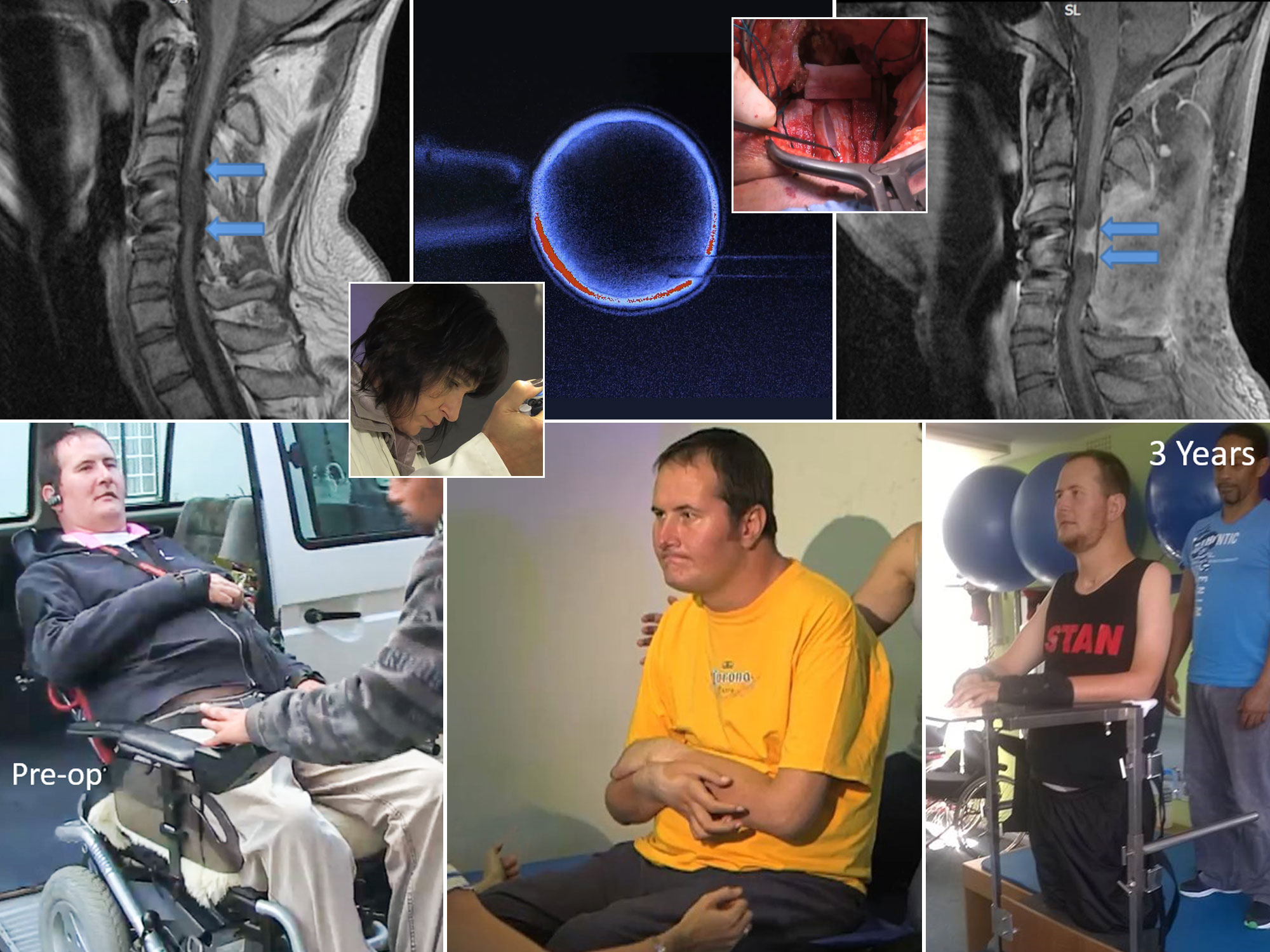
The problem we solve: Accelerating the productization of a new personal (autologous) biological medicine (rebirthed pluripotent stem cells) that regrow neural stem cells in the spinal cord canal to reconnect separated spinal cords in victims of severe chronic spinal cord injury.

About our solution: This stem cell culturing and application process is already proven to work in in-vivo animal and in-vitro human tissue trials and in a compassionate care experimental operation with a human patient in 2012. The process is safe and ethical, and promises to be very affordable.

Progress to date:Ten years of R&D, six of which led to a successful pioneering application with a human patient in 2012. A neurosurgical procedure using these stem cells was approved by the national medical research ethics committee in South Africa in 2015. A clinical trial using these cells will begin in September 2016.
About Our Team

Creator: Anthony Stonefield
Location: New York
Bio: Serial entrepreneur in innovations with 24 years of experience in venture development globally. Head of Healthcare Solutions for an asset management firm in Switzerland and the U.A.E. Biology degree from UC Santa Cruz. Pioneered mobile content business with the first international ringtone business that generating over $1.5 billion in sales. Has raised over $25 million in venture financing for disruptive technology ventures and has led innovation projects for three Fortune 500 companies.
Hospital Affiliation: Frere Hospital
Title: Co-founder & Executive Chairman
LinkedIn: https://za.linkedin.com/in/astonefield
About Team Members
David Anderson
Chief Operating Officer, Bachelor's degree in Chemical Engineering
Biography: Seasoned executive in the pharmaceutical and specialty chemical industries having led several businesses in the US, UK and South Africa, including regional divisions of Ciba-Geigy/Novartis and Rohm & Haas. Successfully started up or turned around several companies. Degree in Chemical Engineering. Five years of local legal, commercial and regulatory review for international stem cell business projects.
Title: Chief Operating Officer
Advanced Degree(s): Bachelor's degree in Chemical Engineering
LinkedIn:
https://za.linkedin.com/in/dave-anderson-539a021
Peter Gees
Chief Legal & Financial Officer, Certificate of Chartered Accounting
Biography: Peter is a Chartered Accountant, a Master Tax Practitioner and an admitted Advocate of the High Court of South Africa. He is the founder of specialized financial-legal consultancy, encompassing 20 years of experience in capital raising, deal corporate finance structuring, IP protection, and developing and implementing cross-border structures, especially in relation to international trade, finance and investment, including compliance, particularly with regard to OECD imperatives.
Title: Chief Legal & Financial Officer
Advanced Degree(s): Certificate of Chartered Accounting
About Our Company

AutoRegenic Cell Laboratories
Location: C/o Horizen Ventures, 161 West 54th Street
602
New York, NY 10019
US
Founded: 2013
Website: http://autoregenic.com
Product Stage: Prototype/MVP
YTD Sales: Working on it
Employees: 5-10
How We Help Patients
Waiting years for a cure for a life-destroying disease is a torture in itself. Many researchers are trying to develop mechanical or external tissue bridges across sections of non-functional spinal cord, or employing chemically induced stem cells. But we believe our more "organic" regenerative approach promises the most comprehensive treatment. With your support we will be able to accelerate the productization of an evolutionary biomedical technique so that our small team can make progress in conducting clinical trials in North America so that a safe and comprehensive treatment can be brought to victims of chronic and severe spinal cord ailments, like quadriplegia, as soon as possible.How We Help Physicians
Neurosurgeons, physicians, physiotherapists and other healthcare workers need not be reminded how devastating for a patient a spinal cord separation accident is, nor what conviction is required in the long-term caring for a quadriplegic patient. Our ability to produce autoregenic cells opens up an experimental treatment for C1-4 quadriplegia using ethically produced (via SCNT), patient-specific rebirthed pre-neural stem cells with which to re-originate sections of cauterized spinal cord. With the support of donors and collaborators, we will soon be able to offer training in the application of autoregenic cells to neurosurgeons and specialist clinicians so they may offer their quadriplegic patients an experimental spinal cord re-origination procedure. Regulations permitting, we are eager to partner with the world’s most skilled and progressive neurosurgeons to accelerate the clinical translation of this innovation.How We Help Hospitals
Once autoregenic cell technique enables hospitals, institutions and medical facilities to introduce a new, high-value product—an experimental treatment for C1-4 quadriplegia using ethical, patient-specific rebirthed pre-neural stem cells to re-originate sections of cauterized spinal cord. Regulations permitting, we are eager to partner with the world’s most progressive institutions to accelerate the clinical translation of this innovation.How We Help Partners
Our biomedical innovation can enable breakthroughs in the research and treatment of severe chronic spinal cord injury. When stem cells are used in cSCI, they are typically adult, allogeneic or induced stem cells which are currently not ideal for a comprehensive or lasting cure. Our technique enables the culturing via SCNT, expansion and differentiation of patient-specific pre-neural stem cells for disease modeling, drug interaction testing, and experimental implantation treatments. We are eager to work with partners to accelerate the clinical translation of this innovation.Innovation Details
Intellectual Property Summary
The novel techniques, procedures and materials we employ in relation to Somatic Cell Nuclear Transfer, pluripotent stem cell differentiation, and implantation media and processes ("Techniques") encompass a set of proprietary processes that are held by the company as trade secrets.
Clinical Information
Technique was refined over 10 years in-vitro and -vivo in 2 stages:
Stage 1 - refined iSCNT to produce cloned animals, demonstrating that embryos derived through this process produced live-birth animals without displaying deleterious effects of heteroplasmy. Animals have been successfully cloned by our team, including King Blue Wildebeest, dairy production cow and Merino sheep.
Stage 2 - harvested the pluripotent stem cells from blastocysts, expanding and differentiate them into pre-neural cells for re-implantation into an excised gap in spinal cords. This process saw the return of full motor functions in a rabbit trial and, subsequently, in the first human quadriplegic patient trial who now, nearly 4 years later, has significant body sensation, some upper body and abdominal muscle mobility, and has been able to stand with assistance for up to 45 minutes;
see http://www.facebook.com/MakingTommieWalk/
Regulatory Status
No application has been made to the FDA to date due to resource constraints. The point of raising financing to productize the Technique is to support, complete and expand upon a clinical trial that is currently spinning up—using the experimental product of our Technique in a research hospital South Africa—so that we may utilize the trial protocols (SOP, cGMP, material sourcing, peer review and third-party validations) plus the results of the clinical trial, to support of an application to the FDA and Canadian Dept. of National Health and Welfare to begin similar clinical trials in North America.
How we will use the funds raised
We aim to raise $45,000 to help rapidly formalize the clinical translation and outreach process which will encompass:
Project management - $10,000
Med-tech and legal research - $15,000
Specialist biomedical & business consultancy - $10,000
Travel/telecom/admin/logistics - $2,500
Web & publicity collateral development - $5,000
Capital raising costs - $2,500
The project is planned to be completed in 90 days and will lead into the company raising commercial and research grant capital to formalize a path to clinical trials in North America and the establishment of the necessary corporate and legal structures and partnerships to begin operating.
Thank You
Nerve damage is very difficult to remedy. Severe C1-4 spinal cord injury, leading to comprehensive quadriplegia, is an expensive life-destroying affliction and is effectively incurable. Quadriplegic life, costing between $4M and $6M to sustain with dignity, can easily lead to destitution or suicide. Such crippling spinal injury is a medical setback and emotional torture that can destroy patients and their personal relationships. Our team is dedicated to expediting the advancement of a set of techniques, materials and processes—for producing re-originated patient-specific stem cells ethically, practically and cost effectively—that is showing encouraging safety and effectiveness for regenerating spinal cord tissue endogenously.
Updates
-
Update #1
ARC implantation clinical trial commences!
We have received the MRIs, Patient Informed Consent declarations, and skin biopsies from the first two patients in the clinical trial. We're approximately halfway done culturing the ARC cells for the first patient -- a 24 year-old man whose injury is just under one year old -- whose operation is scheduled fro October 26th. -
Update #2
Delayed start for first patient
Due to the fact that Frere Hospital has not yet received the growth media required by our team, the first patient operation in the clinical trial has been postponed. The patient's ARC mass is ready. It is expected to take place within the next 10 days. The first patient is a incomplete quadriplegic which add an interesting dimension to the project as scar tissue needs to be extremely carefully excised to preserve those neural paths that are still functional.
Supporters
-
, Master’s Degree in Business Administration from Seton Hall University
10/06/2017 - Followed the project., Master’s Degree in Business Administration from Seton Hall University
10/06/2017 - Liked the project. , MS
, MS
10/25/2016 - Interested in investing in the project. , MS
, MS
10/04/2016 - Backed the project for $1 , MD, MS, MBA
, MD, MS, MBA
08/25/2016 - Liked the project. , PhD in autodidact
, PhD in autodidact
08/24/2016 - Liked the project. , MS
, MS
08/16/2016 - Liked the project. , M.S., B.Tech.
, M.S., B.Tech.
08/10/2016 - Followed the project. , M.S., B.Tech.
, M.S., B.Tech.
08/10/2016 - Liked the project.
08/10/2016 - Followed the project.
08/09/2016 - Liked the project.
08/08/2016 - Followed the project.
08/08/2016 - Liked the project.
08/04/2016 - for the Innovation Supporter
08/04/2016 - Liked the project. , MS
, MS
08/03/2016 - Interested in trying the project.
08/02/2016 - Liked the project.
08/01/2016 - Liked the project.
08/01/2016 - Followed the project.
07/29/2016 - Followed the project.
07/29/2016 - Liked the project.
07/28/2016 - Liked the project.
07/28/2016 - Followed the project.
07/26/2016 - for the Dream Maker
07/26/2016 - for the Pioneer Supporter Instant Feedback
Instant Feedback
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.
27Medstartr
Index Score22
Interest
Score1
Adoption
Score13
Likes0
Partners0
Pilots1
Investors-
This campaign has ended but you can still get involved.See options below.
$11,601 pledged of $45,000 goal$10,000 Investor, Pilot & Parnter
interest to date.
Instant Feedback
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.



