GeneYes: VALIDATE, The Perfect Medical Interviewer
A conversational AI driven animated virtual human platform for collecting, analyzing, and delivering patient reported data to primary care physicians with point of care augmented intelligence.
Boca Raton, FL United States Patient and Provider Tools Connected Health Equity Raise AMIA2019 challengeAbout our project

The problem we solve: Doctor--patient communication has become highly fragmented and inconsistent. Doctors pressed for time often take shortcuts on the data they collect from patients. Therefore, doctors fail to collect detailed, and potentially life-altering information from their patients. The result is care is compromised. For example, most primary care physicians do not collect detailed family histories and therefore do not identify the tens of million of Americans at-risk for hereditary diseases, such as the 18 million at-risk for hereditary cancer.
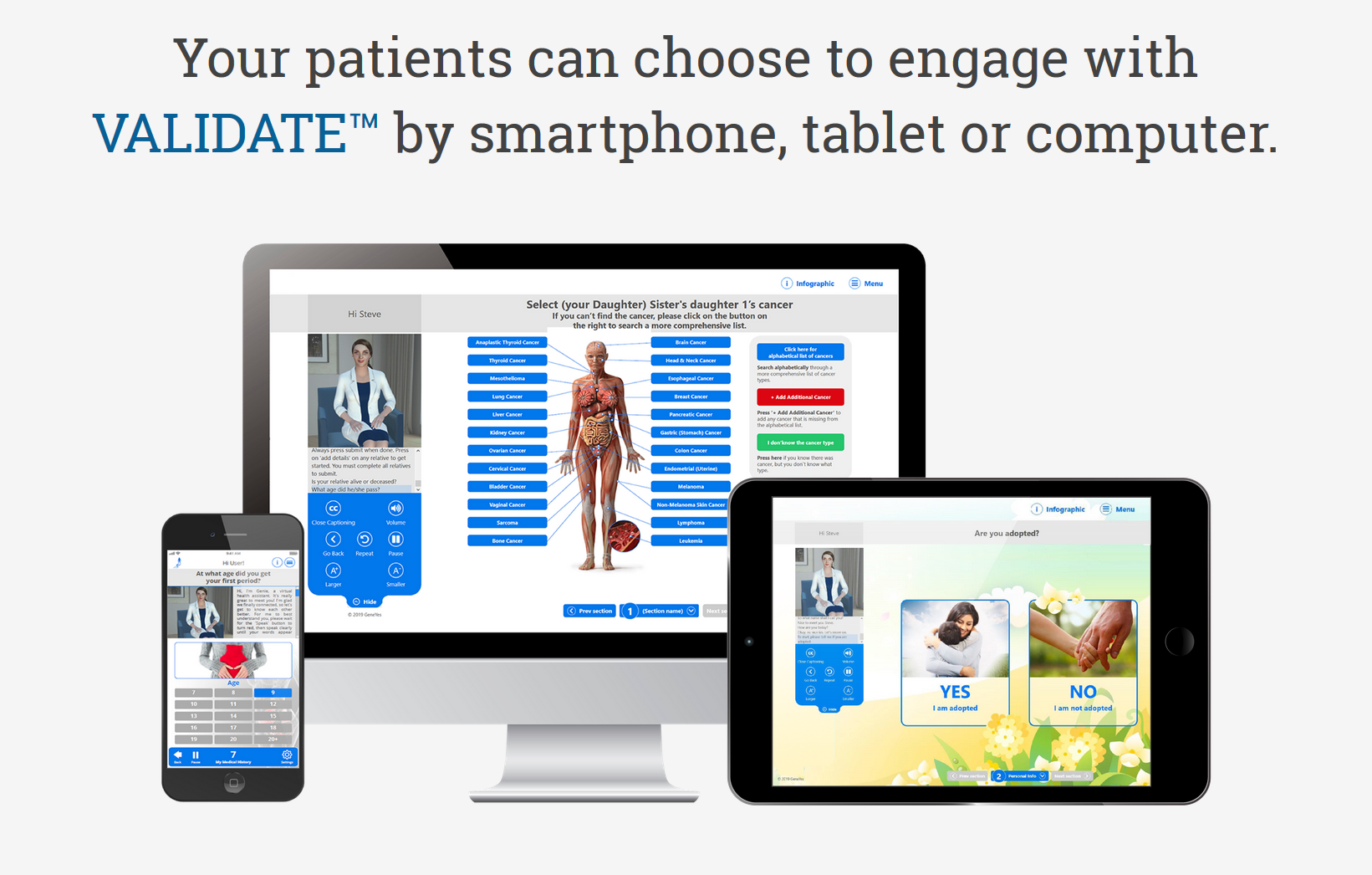
About our solution: We. have created VALIDATE, Virtual Agent inked Intelligent Disease Assessment Tool Engine, the Perfect Medical Interviewer, a full bodied animated conversational AI-driven cloud-based software platform that is both patient and physician friendly. VALIDATE collects a detailed medical history, including a three generation family history, analyzes the data, and deliver the data electronically to the patient's physician with point of care augmented intelligence for clinical decision support accompanied by a new method of illustrating disease risk.
Progress to date:
GeneYes has just completed a fifth-generation web-based version of VALIDATE capable of conversational, rapport-building chit chat, and comprehensive patient reported data collection of a three-generation family cancer and genetics testing history, personal medical history, and ethnicity/ancestry. Using Google speech recognition and Watson assistant, the conversational agent has been highly trained to be quite responsive. For example, if in response to the agent asking the user’s name, the user responds, “My name is William, some call me Will, but I prefer Billy,” the agent will respond, “Nice to meet you Billy.”
If asked “where are you located?” and the user responds “In Washington DC,” the agent will respond in turn that “That’s great, I’ve never been to Washington DC, but it’s on my bucket list.” If instead the user responds to the location question that he or she “is in hell,” the agent will respond, “Cool. Please don’t forget to moisturize.”
When asking sensitive questions such as the sex of the user, the agent will say, “I’m a woman, but I don’t like to make assumptions about someone else’s gender, so can you please tell me if male or female was listed on your original birth certificate?” If user responds to “Yes” to question about is he adopted, agent will respond. “Me too! Did you know there are 135,000 adoption each year in the US.
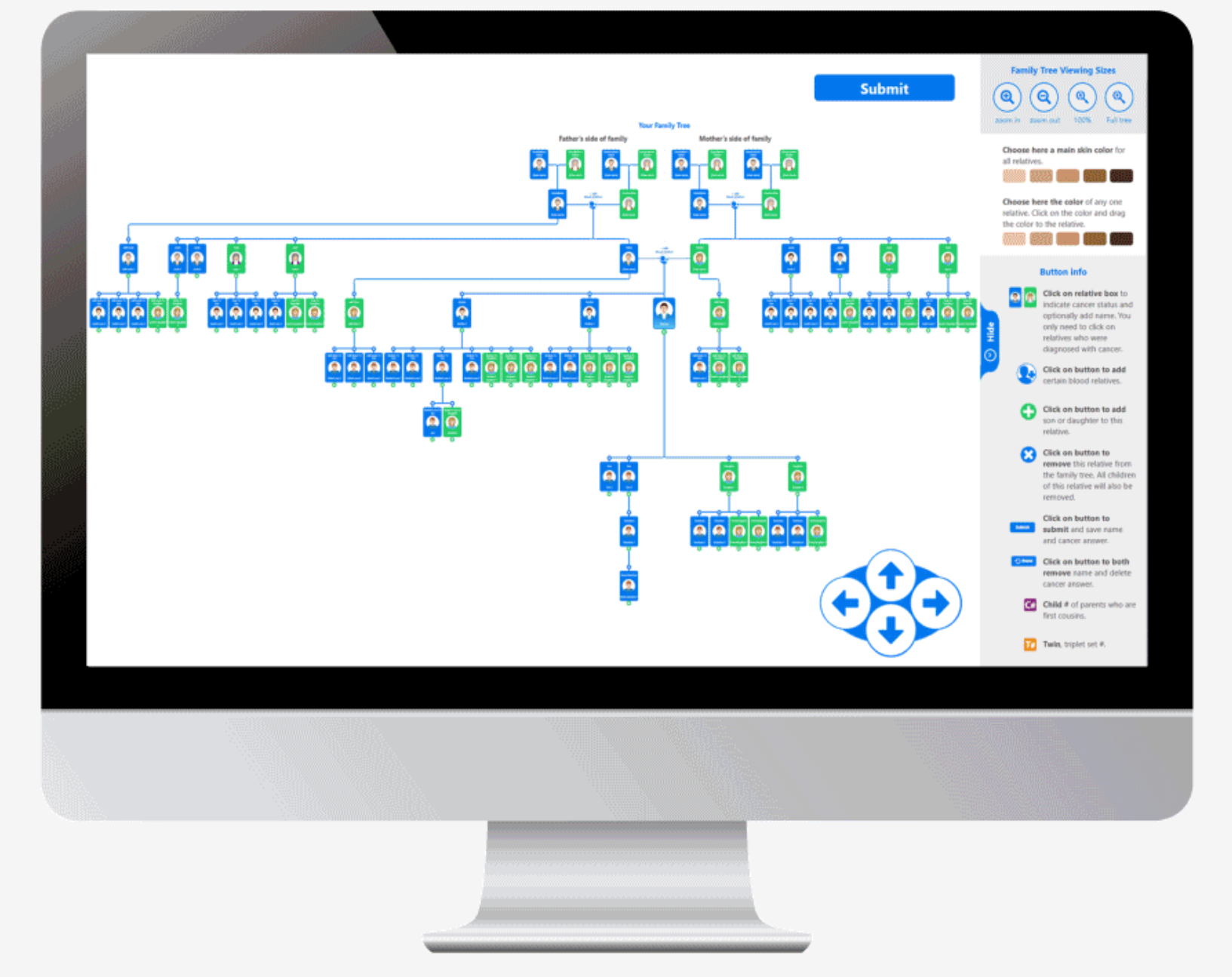
The current version can assemble a 100 person family tree in an average of 4 minutes. The skin color of anyone on the tree or the entire tree can be changed among six different skin colors. Relatives can easily be added or subtracted. The database contains over 1,000 cancers and virtually all medical conditions. Every question is accompanied by a high resolution image that increases intuitiveness of choosing the correct response.
Preliminary data. A second generation version was awarded a 2017 Stanford SPADA grant. A third-generation version of VALIDATE underwent a ten person preliminary study at Brigham and Women’s Hospital in December 2017. The short IRB approved proof of concept study revealed that users enjoyed interacting with the platform and the virtual agent, and demonstrated that VALIDATE was able to identify several relatives and medical conditions missed by the participating Harvard genetic counselors who participated in the study. The platform is now undergoing extensive beta testing in preparation for more extensive research.
About Our Team

Creator: Steven Charlap
Location: Florida
Education: Harvard Business School, NYU School of Medici
Bio: MD, NYU: MBA, Harvard; Fellow, Stanford; co-founder HealthDrive (sold), two-time Inc. 500 company – built first US mobile, multispecialty electronic medical record system; co-founder MDPrevent; T Cell Sciences (public); In Vitro Care (public); Visiting Scientist Harvard
Hospital Affiliation: Harvard
Title: CEO
Advanced Degree(s): MD, MBA
About Team Members
Sinhwa Kang
DIRECTOR OF VIRTUAL HUMANS, PHD
Biography: PhD in Communications, RPI Communications, USC Virtual Agent Researcher
10+ years research experience at USC with virtual agents and human-computer interaction
Title: DIRECTOR OF VIRTUAL HUMANS
Advanced Degree(s): PHD
LinkedIn:
https://www.linkedin.com/in/sinhwa-kang-phd-2957265/
Smruti Vidwans
Chief Science Officer, PHD
Biography: PhD in Genetics, UCSF, CollabRx, McKinsey
8+ years experience as Chief Science Officer at CollabaRX, 2+ at McKinsey, Startup Founder
Title: Chief Science Officer
Advanced Degree(s): PHD
LinkedIn:
https://www.linkedin.com/in/smrutividwans/
Silke Dodel
Director of Computational Sciences, PHD
Biography: PhD in Theoretical Physics, The University of Göttingen, Statistics and Mathematics, Business Calculus, Experimental Design and Computational Social Psychology (adopting Big Data approaches for Natural Language Analysis using R)
Title: Director of Computational Sciences
Advanced Degree(s): PHD
LinkedIn:
https://www.linkedin.com/in/silkedodel/
Ania Knapinska
Director of Data Management, PHD
Biography: PhD in Molecular Biology, Rutgers University, Research and Operations Leader with background in Project Management, Laboratory and Team Leadership
Title: Director of Data Management
Advanced Degree(s): PHD
LinkedIn:
https://www.linkedin.com/in/annaknapinska/
About Our Company

GeneYes Corporation
Location: 901 NW 35th STREET
Boca Raton, FL 33431
US
Founded: 2017
Website: https://www.GeneYes.com
Facebook: https://www.facebook.com/pages/category/Medical---Health/GeneYes-2116631441902115/
Product Stage: Prototype/MVP
YTD Sales: Working on it
Employees: 3-5
How We Help Patients
Hereditary and familial cancer (HFC) may account for up to 33% of all malignancies. In 2018, about 1.8 million new cases of cancer were diagnosed in the United States, representing up to 600,000 cases of HFC. As HFC is identifiable at least 30 years in advance, it is projected that up to 18 million Americans may be at risk. In a 2017 CBS poll, more than half of Americans reported cancer in their family so the number of affected people may be even higher. Collection and assessment of a three-generation family cancer and genetic testing history, personal medical history, and ethnicity (FCGME) is the universally recognized first step to identifying at-risk individuals. Genetic counselors (GCs), who are the gold standard in collecting and assessing FCGME, are in short supply. Numbering less than 5,000, there are too few GCs to screen all those at risk. In addition, GCs depend on our targeted customers, the 230,000+ primary care physicians (PCPs) to whom more than 50% of all medical visits totaling over half a billion are made each year, to identify and refer at-risk patients. To make appropriate referrals, PCPs need to collect and properly assess FCGME. However, FCGME collection and assessment by PCPs is infrequent and of low quality due to many issues facing them including 1. lack of time to collect FCGME, 2. limited training in synthesizing FCGME data into an actionable plan, 3. lack of standardized FCGME collection methods and 4. being unaware of existing reimbursement. As a result, PCPs are under- and over-referring patients for genetic testing and to GCs. Furthermore, up to 92% of patients referred to GCs often do not schedule appointments due to numerous issues, e.g., cost, time, fear of genetic testing, and the referring PCP not clearly explaining the need for referral. Consequently, there is a clear, unmet need to enable PCPs to identify and assess at-risk individuals for better GC referrals to prevent millions of untimely deaths, spare inestimable pain and suffering, and lower treatment costs.
How We Help Physicians
Hereditary and familial cancer (HFC) may account for up to 33% of all malignancies. In 2018, about 1.8 million new cases of cancer were diagnosed in the United States, representing up to 600,000 cases of HFC. As HFC is identifiable at least 30 years in advance, it is projected that up to 18 million Americans may be at risk. In a 2017 CBS poll, more than half of Americans reported cancer in their family so the number of affected people may be even higher. Collection and assessment of a three-generation family cancer and genetic testing history, personal medical history, and ethnicity (FCGME) is the universally recognized first step to identifying at-risk individuals. Genetic counselors (GCs), who are the gold standard in collecting and assessing FCGME, are in short supply. Numbering less than 5,000, there are too few GCs to screen all those at risk. In addition, GCs depend on our targeted customers, the 230,000+ primary care physicians (PCPs) to whom more than 50% of all medical visits totaling over half a billion are made each year, to identify and refer at-risk patients. To make appropriate referrals, PCPs need to collect and properly assess FCGME. However, FCGME collection and assessment by PCPs is infrequent and of low quality due to many issues facing them including 1. lack of time to collect FCGME, 2. limited training in synthesizing FCGME data into an actionable plan, 3. lack of standardized FCGME collection methods and 4. being unaware of existing reimbursement. As a result, PCPs are under- and over-referring patients for genetic testing and to GCs. Furthermore, up to 92% of patients referred to GCs often do not schedule appointments due to numerous issues, e.g., cost, time, fear of genetic testing, and the referring PCP not clearly explaining the need for referral. Consequently, there is a clear, unmet need to enable PCPs to identify and assess at-risk individuals for better GC referrals to prevent millions of untimely deaths, spare inestimable pain and suffering, and lower treatment costs.
GeneYes developed VALIDATE to shift the paradigm of HFC genetic risk assessment (GRA) from a limited number of GCs to the far greater number of PCPs, by explicitly addressing PCP implementation barriers. VALIDATE offers PCPs an immediate solution, avoiding recurrence of what happened with blood chemistry testing in the early 1900s when adoption of testing into routine practice was delayed by many years due to PCPs lack of training and ease with tests. To avoid similar delays with GRA, VALIDATE will accelerate and elevate PCP comfort with GRA providing the “market” with the right solution at the right time to advance primary care clinical genomic practice. The “market’ includes PCPs, who want to prevent HFC but lack time to practice preventive medicine. A tool that collects, assesses and electronically-delivers FCGME data supported by augmented intelligence for clinical decision support will enable PCPs to both identify at-risk patients and increase reimbursement via existing codes.
How We Help Hospitals
Health systems that employ PCPs will financially benefit from downstream revenue generated by providing enhanced surveillance of at-risk patients, e.g. MRIs. More importantly, they will positively impact physicioan morale as physicians perform less work, receive more reimbursement, and provide better patient care.
How We Help Partners
Payers cover genetic tests, but limit approval to specific indications. Many payers currently rely on labor- intensive prior-approval processes to review PCP testing requests. Payers and self-insured employers, our secondary customers, will benefit from a low-cost tool that identifies at-risk individuals.
Challenge Mission
Affiliation(s)
Dr. Charlap is presently a Visiting Scientist at Harvard.
Key Milestones Achieved and Planned
Filed patent, built founding team, received grant from Stanford, completed version 5 of VALIDATE, submitted SBIR to National Cancer Institute (NCI) after completing NCI SBIR Application Mentorship Program, lined up all key clinical resources to perform validation research, and partnered with new development team.
Our Competitive Advantages
Although many tools have been developed to assist PCPs to collect and assess family cancer history, none have achieved wide adoption. Reasons cited include low levels of patient-engagement, lack of clinical validation and utility, under-identified PCP reimbursement opportunities, and poor integration into physician workflows; PCPs refuse to ‘buy?in’ to such programs until they trust and ‘feel’ they can easily work with a tool.
Barriers to Entry
We expect to price our platform at the low end to make competitive entry more difficult and to build out a full suite of clinical modules with multi-lingual, multi-racial characters.
Funding, Partners and Alliances To Date
We have raised $300,000 through Friends and Family and a Stanford grant.
Revenue
There are 5 revenue streams. I will described each briefly: 1. Large Health systems using Epic will pay monthly subscriptions per physician users. 2. Smaller Physician Groups can obtain access directly for a subscription fee 3. Payers can use our system to QA and do the prior approval process for genetic testing 4. Reviews of their genetic testing results for patients for consultation fees 5. Genetic Testing Labs seeking our audiences will pay a marketing fee 6. The Data we collect is unique and can be analyzed for drug studies and other analytics. Our team will partner with organizations that would like to access the data in HIPAA compliant ways to improve outcomes and therapiesInnovation Details
Intellectual Property Summary
Patent filed three years ago, but not yet adjudicated.
Patent Link
https://patents.google.com/patent/US20170300648A1/en
Clinical Information
GeneYes did a 10 person study in December 2017 at Harvard. The study demonstrated that VALIDATE identified relatives missed by genetic counselors and that research subjects enjoted interacting with the charcater and software. The National Cancer Institute (NCI) just mentored us through a application mentorship competitive pilot program for 10 weeks from June through August to submit a SBIR grant application by SEptember 5th to the NCI for $400,000 to complete a series of studies to demonstarte clinical validity, accuracy and utility. The application was accompanied by letters of support from the head of medical research at Stanford, the head of Cancer Prevention at Harvard's Dana Farber, the interim Chief Medical Officer at the American Cancer Society, the Head of Primary Care at NYU and author of the definitive book on medical interviewing, the head of Internal Medicine at the University of Miami, the VP of Innovation at Ascension Healthcare, the Director of the Harvard Innovation Lab, the CEO of the Genetic Alliance, the elad breast cancer researchers at Harvard and Stanford and the lead author of the American College of Genetics and Genomics genetic risk assessment guidelines. They all raved about the product.
Regulatory Status
Our product does not require FDA approval as it does not diagnose. Rather, it flags when data collected matches published guidelines and informs the patient's physician what is the appropriate follow-up.
How we will use the funds raised
The funds will be used to strictly develop a devops team to finalize a commercially sustainable, clinical-grade product and to develop the business development, marketing, and sales team.
Thank You
Six years ago, one of my older brothers, a practicing cardiologist, was diagnosed with two primary cancers. He succumbed 14 months later. His cancer workup revealed he had a BRCA2 mutation, the gene mutation commonly associated with breast and ovarian cancer. This should have come as no surprise given that my father’s niece, our first-cousin, and his two sisters, our aunts, were all diagnosed with early breast cancer in their 30s, 40s, and 50s. In addition, our father was diagnosed with prostate cancer, also associated with the BRCA2 mutation, although later in life in his 70s. But it did surprise us because no physician had ever inquired about his and even till today, my now expansive (12 cases in all) family history of cancer. If a doctor had, and he or she understood the clinical significance of such early cases of breast cancer on one side of the family, that doctor may have instituted increased surveillance, which could have found my brother’s cancers earlier when effective treatment may still have been possible.
Fortunately, I don’t have the mutation, not that any doctor has ever bothered to ask. However, it is ironic that my brother was diagnosed with hereditary cancer while I was the Chief Medical Officer of a primary prevention medical clinic in Florida and was widely touting the impact of lifestyle over genetics in live longer to tens of thousands of people through TV, radio and speaking presentations. My brother’s diagnosis gave me pause to reconsider my attitude toward genetic predispositions and a realization of the need to understand the science better. It also made me really ponder why didn’t doctors collect detailed family medical histories. After my brother passed, I closed my clinic and went to Stanford to pursue gaining that knowledge.
I recently completed a National Science Foundation funded program that allowed me to interview 17 primary care physicians from Florida to New York to California. They described how hard pressed they are for time to collect detailed family histories and perform valid risk assessments. Basically, they shared that very few of them are collecting sufficient family history to fully assess risk, and even among those trying to do so, the doctors admitted they are not doing it well. They collectively all know they need help if the situation is going to change and would be excited to embrace a solution that is clinically validated, integrated into their workflows, and easy for their patients to use.
This is why GeneYes is a mission-driven organization. We want to spare others from the pain and suffering of passing from or losing someone to a preventable or earlier identifiable genetically-driven adult disease such as cancer by enabling genetic risk assessment to take place in the 230,000+ U.S. primary care practices.
Investor Info
Market Size
The virtual health assistant market is rapidly growing if you can beleive all the companies releasing market reports. Clinically, every American should receive genetic risk assessment based on family and personal medical history, and lifestyle and environental exposures. Depending on how we price annual usage of our first use case, herediatry cancer, we estimate the market at $1.5 billion at a $5 pppu and above. We have identified dozens of use cases so we beleive this represents a $100 billion opportunity across all health care use cases, including pharamaceutical drug trials, clinical research and clinical usage.
Projected 3 Year Growth
For our first use case, hereditary cancer risk assessment, we estimate the total US addressable market (UTAM) as approximately $4.5 billion. We estimate the UTAM for overall genetic risk assessment, including cardiology, stroke, diabetes, epigenomics, nutrigenomics, pharmacogenomics, etc. to be north of $50 billion, and the UTAM for patient reported data collection and analysis to grow to $1 trillion by 2030. This does not include worldwide estimates.
>We expect to generate three million in revenue, or .3% of the market, by year three, with a margin of 40% or $1.2. in EBITDA.
Revenue Model
We see our primary customers as health systems as well as payors such as CMS. Provider systems need to protect their physicians' mental health and productivity, while their docs try to stay current with medical advances. Our business model is expected to be subscription-based and should be more than adequately covered by increased physician reimbursement associated enabling raising of Evaluation & Management code levels with the addition of genetic risk assessment that includes more detailed histories and more complicated medical decision-making, and by generating downstream revenue for health systems by increased billings, e.g. MRIs, colonoscopies, genetic testing, lab work, surgeries, etc.
Insurance payers, who are actively trying to control expenses related to genetic testing, are also expected customers. These payers currently either use expensive and in short supply genetic counselors on staff or pay companies like AIM Specialty Health to pay companies like InformedDNA and Genome Medical to review physician orders for testing. VALIDATE can significantly reduce their costs to a fraction of what they are paying now, which can total in the hundreds of dollars.
With growing interest in personal health records, we plan to attract consumer subscribers who sign up after using the platform for their physicians for additional value-added services, e.g. never completing a health form again, reclassification of variants on a routine basis, risk analysis, etc.
The data captured by our platform is unique because it links generational and personal health data that falls outside the purview of individuals EMR data. As such, we imagine that our data would be of interest to researchers - in pharma, academic and clinical institutions. VALIDATE will also be useful for clinical trials for collecting patient reported data and outcomes.
We also believe that the highly competitive genetic testing lab market will be interested in paying for access to our users.
Competitors
Our most common competitor is pen and paper. There are several for-profit companies, e.g. CancerIQ, Clear Genetics, Igentify, SmartGenome, Progeny, etc. and governmental/non-profit related efforts, e.g. All of US, the Million Vets Project, MeTree, etc. underway that do data collection that overlaps with our own. Most are focused on either simply automating data collection or decreasing reliance on genetic counselors. But not a single competitor currently offers a primary care-focused solution that innovates patient reported data collection with a comprehensive, clinically robust, and conversationally advanced technology as VALIDATE.
Our competitive advantages are:
- A focus on perfecting the art and science of medical interviewing by combining the best of human and computer capabilities.
- A patent-pending end-to-end solution for collecting, analyzing, delivering, and illustrating disease risk>
- A well-balanced team with deep expertise in entrepreneurship, clinical, genomics, virtual agents, and conversational AI technology
Traction
Many potential customers, Harvard, Stanford, Univ. of Miami, ACOG, ACS, etc. have indicated interest after viewing demos. All are waiting for clinicla validation studies to be completed.
Due Diligence Docs
Please note that access to the company's confidential materials is limited. Click this button to request access from the Company and its representatives.
Updates
No updates found .
Supporters
-
, DO
11/05/2019 - Liked the project.
11/04/2019 - Liked the project.
10/27/2019 - Liked the project.
10/25/2019 - Liked the project.
10/21/2019 - Liked the project.
10/07/2019 - Followed the project.
10/07/2019 - Followed the project.
10/05/2019 - Followed the project.
10/02/2019 - Liked the project.
10/02/2019 - Followed the project.
10/01/2019 - Followed the project.
10/01/2019 - Liked the project.
09/27/2019 - Followed the project.
09/27/2019 - Liked the project.
09/27/2019 - Liked the project.
09/27/2019 - Followed the project.
09/26/2019 - Liked the project. , MD, MBA
, MD, MBA
09/26/2019 - Followed the project. , MD, MBA
, MD, MBA
09/26/2019 - Liked the project.
09/26/2019 - Liked the project.
09/26/2019 - Followed the project.
09/26/2019 - Followed the project.
09/26/2019 - Liked the project.
09/26/2019 - Liked the project.
09/26/2019 - Followed the project.
09/26/2019 - Liked the project.
09/26/2019 - Followed the project.
09/26/2019 - Liked the project.
09/26/2019 - Liked the project.
09/26/2019 - Followed the project.
09/26/2019 - Liked the project.
09/26/2019 - Followed the project.
09/26/2019 - Followed the project.
09/26/2019 - Liked the project.
09/26/2019 - Followed the project.
09/26/2019 - Liked the project.Instant Feedback
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.
86Medstartr
Index Score86
Interest
Score0
Adoption
Score22
Likes0
Partners0
Pilots18
Follows-
This campaign has ended but you can still get involved.See options below.
$ 1,000,000 goal
Instant Feedback
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.